NRF2 Promotes Tumor Maintenance by Modulating mRNA Translation in Pancreatic Cancer
- PMID: 27477511
- PMCID: PMC5234705
- DOI: 10.1016/j.cell.2016.06.056
NRF2 Promotes Tumor Maintenance by Modulating mRNA Translation in Pancreatic Cancer
Abstract
Pancreatic cancer is a deadly malignancy that lacks effective therapeutics. We previously reported that oncogenic Kras induced the redox master regulator Nfe2l2/Nrf2 to stimulate pancreatic and lung cancer initiation. Here, we show that NRF2 is necessary to maintain pancreatic cancer proliferation by regulating mRNA translation. Specifically, loss of NRF2 led to defects in autocrine epidermal growth factor receptor (EGFR) signaling and oxidation of specific translational regulatory proteins, resulting in impaired cap-dependent and cap-independent mRNA translation in pancreatic cancer cells. Combined targeting of the EGFR effector AKT and the glutathione antioxidant pathway mimicked Nrf2 ablation to potently inhibit pancreatic cancer ex vivo and in vivo, representing a promising synthetic lethal strategy for treating the disease.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
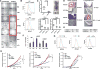

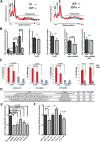




Comment in
-
Pancreatic cancer: A role for redox regulation in PDAC.Nat Rev Gastroenterol Hepatol. 2016 Aug 24;13(9):498. doi: 10.1038/nrgastro.2016.133. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27555516 No abstract available.
References
-
- Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. - PubMed
-
- Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Current opinion in structural biology. 2004;14:679–686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

