UBQLN2 Mediates Autophagy-Independent Protein Aggregate Clearance by the Proteasome
- PMID: 27477512
- PMCID: PMC5003816
- DOI: 10.1016/j.cell.2016.07.001
UBQLN2 Mediates Autophagy-Independent Protein Aggregate Clearance by the Proteasome
Abstract
Clearance of misfolded and aggregated proteins is central to cell survival. Here, we describe a new pathway for maintaining protein homeostasis mediated by the proteasome shuttle factor UBQLN2. The 26S proteasome degrades polyubiquitylated substrates by recognizing them through stoichiometrically bound ubiquitin receptors, but substrates are also delivered by reversibly bound shuttles. We aimed to determine why these parallel delivery mechanisms exist and found that UBQLN2 acts with the HSP70-HSP110 disaggregase machinery to clear protein aggregates via the 26S proteasome. UBQLN2 recognizes client-bound HSP70 and links it to the proteasome to allow for the degradation of aggregated and misfolded proteins. We further show that this process is active in the cell nucleus, where another system for aggregate clearance, autophagy, does not act. Finally, we found that mutations in UBQLN2, which lead to neurodegeneration in humans, are defective in chaperone binding, impair aggregate clearance, and cause cognitive deficits in mice.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



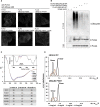







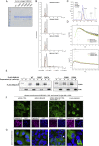



Comment in
-
Look Out Autophagy, Ubiquilin UPS Its Game.Cell. 2016 Aug 11;166(4):797-799. doi: 10.1016/j.cell.2016.07.048. Cell. 2016. PMID: 27518558
References
-
- Bennett E.J., Bence N.F., Jayakumar R., Kopito R.R. Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol. Cell. 2005;17:351–365. - PubMed
-
- Bett J.S., Goellner G.M., Woodman B., Pratt G., Rechsteiner M., Bates G.P. Proteasome impairment does not contribute to pathogenesis in R6/2 Huntington’s disease mice: exclusion of proteasome activator REGgamma as a therapeutic target. Hum. Mol. Genet. 2006;15:33–44. - PubMed
-
- Cornett J., Cao F., Wang C.-E., Ross C.A., Bates G.P., Li S.-H., Li X.-J. Polyglutamine expansion of huntingtin impairs its nuclear export. Nat. Genet. 2005;37:198–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

