Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study
- PMID: 27477525
- PMCID: PMC5570461
- DOI: 10.1016/j.eururo.2016.07.032
Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study
Abstract
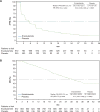
Enzalutamide significantly improved radiographic progression-free survival (rPFS) and overall survival (OS) among men with chemotherapy-naïve metastatic castration-resistant prostate cancer at the prespecified interim analysis of PREVAIL, a phase 3, double-blind, randomized study. We evaluated the longer-term efficacy and safety of enzalutamide up to the prespecified number of deaths in the final analysis, which included an additional 20 mo of follow-up for investigator-assessed rPFS, 9 mo of follow-up for OS, and 4 mo of follow-up for safety. Enzalutamide reduced the risk of radiographic progression or death by 68% (hazard ratio [HR] 0.32, 95% confidence interval [CI] 0.28-0.37; p<0.0001) and the risk of death by 23% (HR 0.77, 95% CI 0.67-0.88; p=0.0002). Median investigator-assessed rPFS was 20.0 mo (95% CI 18.9-22.1) in the enzalutamide arm and 5.4 mo (95% CI 4.1-5.6) in the placebo arm. Median OS was 35.3 mo (95% CI 32.2-not yet reached) in the enzalutamide arm and 31.3 mo (95% CI 28.8-34.2) in the placebo arm. At the time of the OS analysis, 167 patients in the placebo arm had crossed over to receive enzalutamide. The most common adverse events in the enzalutamide arm were fatigue, back pain, constipation, and arthralgia. This final analysis of PREVAIL provides more complete assessment of the clinical benefit of enzalutamide. PREVAIL is registered on ClinicalTrials.gov as NCT01212991.
Patient summary: According to data from longer follow-up, enzalutamide continued to provide benefit over placebo in patients with metastatic castration-resistant prostate cancer.
Keywords: Androgen receptor signaling inhibitor; Enzalutamide; Metastatic castration-resistant prostate cancer; Overall survival; Radiographic progression-free survival.
Copyright © 2016 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical