Inhibition of the prostaglandin E2 receptor EP2 prevents status epilepticus-induced deficits in the novel object recognition task in rats
- PMID: 27477533
- PMCID: PMC5028311
- DOI: 10.1016/j.neuropharm.2016.07.028
Inhibition of the prostaglandin E2 receptor EP2 prevents status epilepticus-induced deficits in the novel object recognition task in rats
Abstract
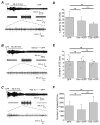
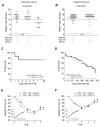
Survivors of exposure to an organophosphorus nerve agent may develop a number of complications including long-term cognitive deficits (Miyaki et al., 2005; Nishiwaki et al., 2001). We recently demonstrated that inhibition of the prostaglandin E2 receptor, EP2, attenuates neuroinflammation and neurodegeneration caused by status epilepticus (SE) induced by the soman analog, diisopropylfluorophosphate (DFP), which manifest within hours to days of the initial insult. Here, we tested the hypothesis that DFP exposure leads to a loss of cognitive function in rats that is blocked by early, transient EP2 inhibition. Adult male Sprague-Dawley rats were administered vehicle or the competitive EP2 antagonist, TG6-10-1, (ip) at various times relative to DFP-induced SE. DFP administration resulted in prolonged seizure activity as demonstrated by cortical electroencephalography (EEG). A single intraperitoneal injection of TG6-10-1 or vehicle 1 h prior to DFP did not alter the development of seizures, the latency to SE or the duration of SE. Rats administered six injections of TG6-10-1 starting 90 min after the onset of DFP-induced SE could discriminate between a novel and familiar object 6-12 weeks after SE, unlike vehicle treated rats which showed no preference for the novel object. By contrast, behavioral changes in the light-dark box and open field assays were not affected by TG6-10-1. Delayed mortality after DFP was also unaffected by TG6-10-1. Thus, selective inhibition of the EP2 receptor may prevent SE-induced memory impairment in rats caused by exposure to a high dose of DFP.
Keywords: Anxiety; DFP; EP2 receptor; Electroencephalography; Epilepsy; Hippocampus; Light-dark box preference test; Novel object recognition; Open field; Organophosphate; Seizure; Status epilepticus.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures



References
-
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat Protoc. 2006;1:1306–1311. - PubMed
-
- Binukumar BK, Bal A, Gill KD. Chronic dichlorvos exposure: microglial activation, proinflammatory cytokines and damage to nigrostriatal dopaminergic system. Neuromolecular Med. 2011;13:251–265. - PubMed
-
- Chen Y. Organophosphate-induced brain damage: mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. Neurotoxicology. 2012;33:391–400. - PubMed
-
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

