H3K27me3 Does Not Orchestrate the Expression of Lineage-Specific Markers in hESC-Derived Hepatocytes In Vitro
- PMID: 27477635
- PMCID: PMC4982990
- DOI: 10.1016/j.stemcr.2016.06.013
H3K27me3 Does Not Orchestrate the Expression of Lineage-Specific Markers in hESC-Derived Hepatocytes In Vitro
Abstract
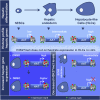
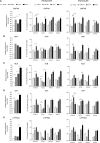
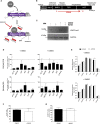
Although pluripotent stem cells can be differentiated into the hepatocyte lineages, such cells retain an immature phenotype. As the chromatin state of regulatory regions controls spatiotemporal gene expression during development, we evaluated changes in epigenetic histone marks in lineage-specific genes throughout in vitro hepatocyte differentiation from human embryonic stem cells (hESCs). Active acetylation and methylation marks at promoters and enhancers correlated with progressive changes in gene expression. However, repression-associated H3K27me3 marks at these control regions showed an inverse correlation with gene repression during transition from hepatic endoderm to a hepatocyte-like state. Inhibitor of Enhancer of Zeste Homolog 2 (EZH2) reduced H3K27me3 decoration but did not improve hepatocyte maturation. Thus, H3K27me3 at regulatory regions does not regulate transcription and appears dispensable for hepatocyte lineage differentiation of hESCs in vitro.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Aoki R., Chiba T., Miyagi S., Negishi M., Konuma T., Taniguchi H., Ogawa M., Yokosuka O., Iwama A. The polycomb group gene product Ezh2 regulates proliferation and differentiation of murine hepatic stem/progenitor cells. J. Hepatol. 2010;52:854–863. - PubMed
-
- Azuara V., Perry P., Sauer S., Spivakov M., Jorgensen H.F., John R.M., Gouti M., Casanova M., Warnes G., Merkenschlager M. Chromatin signatures of pluripotent cell lines. Nat. Cell Biol. 2006;8:532–538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

