Redox-based therapeutics in neurodegenerative disease
- PMID: 27477685
- PMCID: PMC5446580
- DOI: 10.1111/bph.13551
Redox-based therapeutics in neurodegenerative disease
Abstract
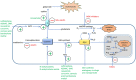
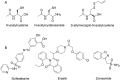
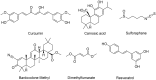
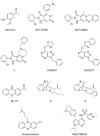
This review describes recent developments in the search for effective therapeutic agents that target redox homeostasis in neurodegenerative disease. The disruption to thiol redox homeostasis in Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis and multiple sclerosis is discussed, together with the experimental strategies that are aimed at preventing, or at least minimizing, oxidative damage in these diseases. Particular attention is given to the potential of increasing antioxidant capacity by targeting the Nrf2 pathway, the development of inhibitors of NADPH oxidases that are likely candidates for clinical use, together with strategies to reduce nitrosative stress and mitochondrial dysfunction. We describe the shortcomings of compounds that hinder their progression to the clinic and evaluate likely avenues for future research.
Linked articles: This article is part of a themed section on Redox Biology and Oxidative Stress in Health and Disease. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.12/issuetoc.
© 2016 The British Pharmacological Society.
Figures
Similar articles
-
NADPH oxidases in oxidant production by microglia: activating receptors, pharmacology and association with disease.Br J Pharmacol. 2017 Jun;174(12):1733-1749. doi: 10.1111/bph.13425. Epub 2016 Feb 26. Br J Pharmacol. 2017. PMID: 26750203 Free PMC article. Review.
-
Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function.Br J Pharmacol. 2017 Jun;174(12):1670-1689. doi: 10.1111/bph.13403. Epub 2016 Feb 4. Br J Pharmacol. 2017. PMID: 26660451 Free PMC article. Review.
-
Microglia antioxidant systems and redox signalling.Br J Pharmacol. 2017 Jun;174(12):1719-1732. doi: 10.1111/bph.13426. Epub 2016 Mar 3. Br J Pharmacol. 2017. PMID: 26754582 Free PMC article. Review.
-
NADPH Oxidases in Neurodegenerative Disorders: Mechanisms and Therapeutic Opportunities.Antioxid Redox Signal. 2024 Sep;41(7-9):522-541. doi: 10.1089/ars.2023.0002. Epub 2024 Jul 10. Antioxid Redox Signal. 2024. PMID: 38760935 Review.
-
ROS in gastrointestinal inflammation: Rescue Or Sabotage?Br J Pharmacol. 2017 Jun;174(12):1704-1718. doi: 10.1111/bph.13428. Epub 2016 Mar 3. Br J Pharmacol. 2017. PMID: 26758851 Free PMC article. Review.
Cited by
-
Accelerated Aging and Age-Related Diseases (CVD and Neurological) Due to Air Pollution and Traffic Noise Exposure.Int J Mol Sci. 2021 Feb 28;22(5):2419. doi: 10.3390/ijms22052419. Int J Mol Sci. 2021. PMID: 33670865 Free PMC article. Review.
-
The Evaluation of Oxidative Stress Parameters in Serum Patients with Relapsing-Remitting Multiple Sclerosis Treated with II-Line Immunomodulatory Therapy.Oxid Med Cell Longev. 2017;2017:9625806. doi: 10.1155/2017/9625806. Epub 2017 Sep 12. Oxid Med Cell Longev. 2017. PMID: 29138683 Free PMC article. Clinical Trial.
-
The Potential Roles of Redox Enzymes in Alzheimer's Disease: Focus on Thioredoxin.ASN Neuro. 2021 Jan-Dec;13:1759091421994351. doi: 10.1177/1759091421994351. ASN Neuro. 2021. PMID: 33557592 Free PMC article. Review.
-
Natural phytochemicals that affect autophagy in the treatment of oral diseases and infections: A review.Front Pharmacol. 2022 Aug 25;13:970596. doi: 10.3389/fphar.2022.970596. eCollection 2022. Front Pharmacol. 2022. PMID: 36091810 Free PMC article. Review.
-
A potent phosphodiester Keap1-Nrf2 protein-protein interaction inhibitor as the efficient treatment of Alzheimer's disease.Redox Biol. 2023 Aug;64:102793. doi: 10.1016/j.redox.2023.102793. Epub 2023 Jun 24. Redox Biol. 2023. PMID: 37385075 Free PMC article.
References
-
- Akterin S, Cowburn RF, Miranda‐Vizuete A, Jimenez A, Bogdanovic N, Winblad B et al. (2006). Involvement of glutaredoxin‐1 and thioredoxin‐1 in beta‐amyloid toxicity and Alzheimer's disease. Cell Death Differ 13: 1454–1465. - PubMed
-
- Albano R, Liu X, Lobner D (2013). Regulation of system x(c)‐ in the SOD1‐G93 A mouse model of ALS. Exp Neurol 250: 69–73. - PubMed
-
- Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I et al. (2008a). Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9: 686–696. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

