Anti-hepatitis C virus potency of a new autophagy inhibitor using human liver slices model
- PMID: 27478540
- PMCID: PMC4958700
- DOI: 10.4254/wjh.v8.i21.902
Anti-hepatitis C virus potency of a new autophagy inhibitor using human liver slices model
Abstract
Aim: To evaluate the antiviral potency of a new anti-hepatitis C virus (HCV) antiviral agent targeting the cellular autophagy machinery.
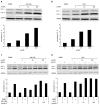
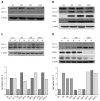
Methods: Non-infected liver slices, obtained from human liver resection and cut in 350 μm-thick slices (2.7 × 10(6) cells per slice) were infected with cell culture-grown HCV Con1b/C3 supernatant (multiplicity of infection = 0.1) cultivated for up to ten days. HCV infected slices were treated at day 4 post-infection with GNS-396 for 6 d at different concentrations. HCV replication was evaluated by strand-specific real-time quantitative reverse transcription - polymerase chain reaction. The infectivity titers of supernatants were evaluated by foci formation upon inoculation into naive Huh-7.5.1 cells. The cytotoxic effect of the drugs was evaluated by lactate dehydrogenase leakage assays.
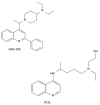
Results: The antiviral efficacy of a new antiviral drug, GNS-396, an autophagy inhibitor, on HCV infection of adult human liver slices was evidenced in a dose-dependent manner. At day 6 post-treatment, GNS-396 EC50 was 158 nmol/L without cytotoxic effect (compared to hydroxychloroquine EC50 = 1.17 μmol/L).
Conclusion: Our results demonstrated that our ex vivo model is efficient for evaluation the potency of autophagy inhibitors, in particular a new quinoline derivative GNS-396 as antiviral could inhibit HCV infection in a dose-dependent manner without cytotoxic effect.
Keywords: Autophagy; Hepatitis C virus; Host antiviral therapy; Quinoline derivative; Tissue culture.
Figures




References
-
- Szabó E, Lotz G, Páska C, Kiss A, Schaff Z. Viral hepatitis: new data on hepatitis C infection. Pathol Oncol Res. 2003;9:215–221. - PubMed
-
- Corouge M, Pol S. New treatments for chronic hepatitis C virus infection. Med Mal Infect. 2011;41:579–587. - PubMed
-
- Mallet V, Gilgenkrantz H, Serpaggi J, Verkarre V, Vallet-Pichard A, Fontaine H, Pol S. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149:399–403. - PubMed
-
- López-Labrador FX. Hepatitis C Virus NS3/4A Protease Inhibitors. Recent Pat Antiinfect Drug Discov. 2008;3:157–167. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

