Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice
- PMID: 27478544
- PMCID: PMC4958640
- DOI: 10.4162/nrp.2016.10.4.386
Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice
Erratum in
-
Erratum: Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice.Nutr Res Pract. 2017 Oct;11(5):435. doi: 10.4162/nrp.2017.11.5.435. Epub 2017 Sep 28. Nutr Res Pract. 2017. PMID: 28989581 Free PMC article.
Abstract
Background/objectives: Changes in nutritional status during gestation and lactation have detrimental effects on offspring metabolism. Several animal studies have shown that maternal high-fat diet (HFD) can predispose the offspring to development of obesity and metabolic diseases, however the mechanisms underlying these transgenerational effects are poorly understood. Therefore, we examined the effect of maternal HFD consumption on metabolic phenotype and hepatic expression of involved genes in dams to determine whether any of these parameters were associated with the metabolic outcomes in the offspring.
Materials/methods: Female C57BL/6 mice were fed a low-fat diet (LFD: 10% calories from fat) or a high-fat diet (HFD: 45% calories from fat) for three weeks before mating, and during pregnancy and lactation. Dams and their male offspring were studied at weaning.
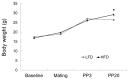
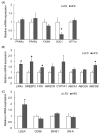
Results: Dams fed an HFD had significantly higher body and adipose tissue weights and higher serum triglyceride and cholesterol levels than dams fed an LFD. Hepatic lipid levels and mRNA levels of genes involved in lipid metabolism, including LXRα, SREBP-2, FXR, LDLR, and ABCG8 were significantly changed by maternal HFD intake. Significantly lower total liver DNA and protein contents were observed in dams fed an HFD, implicating the disturbed liver adaptation in the pregnancy-related metabolic demand. HFD feeding also induced significant oxidative stress in serum and liver of dams. Offspring of dams fed an HFD had significantly higher serum cholesterol levels, which were negatively correlated with liver weights of dams and positively correlated with hepatic lipid peroxide levels in dams.
Conclusions: Maternal HFD consumption induced metabolic dysfunction, including altered liver growth and oxidative stress in dams, which may contribute to the disturbed cholesterol homeostasis in the early life of male mice offspring.
Keywords: Cholesterol metabolism; high-fat diet; liver; offspring; oxidative stress.
Figures






Similar articles
-
Oxidative Stress Profile of Mothers and Their Offspring after Maternal Consumption of High-Fat Diet in Rodents: A Systematic Review and Meta-Analysis.Oxid Med Cell Longev. 2021 Nov 24;2021:9073859. doi: 10.1155/2021/9073859. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34868458 Free PMC article.
-
Leucine supplementation in maternal high-fat diet alleviated adiposity and glucose intolerance of adult mice offspring fed a postweaning high-fat diet.Lipids Health Dis. 2023 Apr 15;22(1):50. doi: 10.1186/s12944-023-01812-4. Lipids Health Dis. 2023. PMID: 37061742 Free PMC article.
-
Pre-Weaning Exposure to Maternal High-Fat Diet Is a Critical Developmental Window for Programming the Metabolic System of Offspring in Mice.Front Endocrinol (Lausanne). 2022 Feb 10;13:816107. doi: 10.3389/fendo.2022.816107. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35222275 Free PMC article.
-
Maternal exposure to high-fat diet during pregnancy and lactation predisposes normal weight offspring mice to develop hepatic inflammation and insulin resistance.Physiol Rep. 2021 Mar;9(6):e14811. doi: 10.14814/phy2.14811. Physiol Rep. 2021. PMID: 33769706 Free PMC article.
-
Could parental high-fat intake program the reproductive health of male offspring? A review.Crit Rev Food Sci Nutr. 2023;63(14):2074-2081. doi: 10.1080/10408398.2021.1970509. Epub 2021 Aug 26. Crit Rev Food Sci Nutr. 2023. PMID: 34445915 Review.
Cited by
-
Inclusion of microbe-derived antioxidant during pregnancy and lactation attenuates high-fat diet-induced hepatic oxidative stress, lipid disorders, and NLRP3 inflammasome in mother rats and offspring.Food Nutr Res. 2019 Aug 23;63. doi: 10.29219/fnr.v63.3504. eCollection 2019. Food Nutr Res. 2019. PMID: 34104129 Free PMC article.
-
Oxidative Stress Profile of Mothers and Their Offspring after Maternal Consumption of High-Fat Diet in Rodents: A Systematic Review and Meta-Analysis.Oxid Med Cell Longev. 2021 Nov 24;2021:9073859. doi: 10.1155/2021/9073859. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34868458 Free PMC article.
-
Leucine supplementation in maternal high-fat diet alleviated adiposity and glucose intolerance of adult mice offspring fed a postweaning high-fat diet.Lipids Health Dis. 2023 Apr 15;22(1):50. doi: 10.1186/s12944-023-01812-4. Lipids Health Dis. 2023. PMID: 37061742 Free PMC article.
-
Nurturing through Nutrition: Exploring the Role of Antioxidants in Maternal Diet during Pregnancy to Mitigate Developmental Programming of Chronic Diseases.Nutrients. 2023 Oct 31;15(21):4623. doi: 10.3390/nu15214623. Nutrients. 2023. PMID: 37960276 Free PMC article. Review.
-
Gender-divergent expression of lipid and bile acid metabolism related genes in adult mice offspring of dams fed a high-fat diet.J Biosci. 2018 Jun;43(2):329-337. J Biosci. 2018. PMID: 29872021
References
-
- Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010;30:315–339. - PubMed
-
- Cianfarani S, Agostoni C, Bedogni G, Berni Canani R, Brambilla P, Nobili V, Pietrobelli A. Effect of intrauterine growth retardation on liver and long-term metabolic risk. Int J Obes (Lond) 2012;36:1270–1277. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

