A Simple, Fast, Low Cost, HPLC/UV Validated Method for Determination of Flutamide: Application to Protein Binding Studies
- PMID: 27478788
- PMCID: PMC4961984
- DOI: 10.15171/apb.2016.034
A Simple, Fast, Low Cost, HPLC/UV Validated Method for Determination of Flutamide: Application to Protein Binding Studies
Abstract
Purpose: The main goal of this study was development of a reverse phase high performance liquid chromatography (RP-HPLC) method for flutamide quantitation which is applicable to protein binding studies.
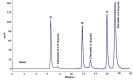
Methods: Ultrafilteration method was used for protein binding study of flutamide. For sample analysis, flutamide was extracted by a simple and low cost extraction method using diethyl ether and then was determined by HPLC/UV. Acetanilide was used as an internal standard. The chromatographic system consisted of a reversed-phase C8 column with C8 pre-column, and the mobile phase of a mixture of 29% (v/v) methanol, 38% (v/v) acetonitrile and 33% (v/v) potassium dihydrogen phosphate buffer (50 mM) with pH adjusted to 3.2.
Results: Acetanilide and flutamide were eluted at 1.8 and 2.9 min, respectively. The linearity of method was confirmed in the range of 62.5-16000 ng/ml (r(2) > 0.99). The limit of quantification was shown to be 62.5 ng/ml. Precision and accuracy ranges found to be (0.2-1.4%, 90-105%) and (0.2-5.3 %, 86.7-98.5 %) respectively. Acetanilide and flutamide capacity factor values of 1.35 and 2.87, tailing factor values of 1.24 and 1.07 and resolution values of 1.8 and 3.22 were obtained in accordance with ICH guidelines.
Conclusion: Based on the obtained results a rapid, precise, accurate, sensitive and cost-effective analysis procedure was proposed for quantitative determination of flutamide.
Keywords: Acetanilide; Flutamide; High pressure liquid chromatography; ICH guidelines; Protein binding.
Similar articles
-
Analytical Method Validation of High-Performance Liquid Chromatography and Stability-Indicating Study of Medroxyprogesterone Acetate Intravaginal Sponges.Anal Chem Insights. 2017 Feb 20;12:1177390117690152. doi: 10.1177/1177390117690152. eCollection 2017. Anal Chem Insights. 2017. PMID: 28469407 Free PMC article.
-
Development and application of an HPLC method for erlotinib protein binding studies.Adv Pharm Bull. 2013;3(2):289-93. doi: 10.5681/apb.2013.047. Epub 2013 Aug 20. Adv Pharm Bull. 2013. PMID: 24312850 Free PMC article.
-
Stereoselective determination of unchanged and glucuroconjugated eliprodil, a new anti-ischaemic drug, in human plasma and urine by precolumn derivatization and column-switching high-performance liquid chromatography with fluorescence detection.J Chromatogr A. 1996 Apr 5;729(1-2):323-33. doi: 10.1016/0021-9673(95)00893-4. J Chromatogr A. 1996. PMID: 9004957 Clinical Trial.
-
Fast RP-HPLC Method for the Determination of Bisoprolol.Rev Med Chir Soc Med Nat Iasi. 2016 Jul-Sep;120(3):720-6. Rev Med Chir Soc Med Nat Iasi. 2016. PMID: 30152661
-
New RP-HPLC Method Development and Validation for Dorzolamide in Ophthalmic Dosage Form.J Anal Methods Chem. 2018 Sep 12;2018:4596141. doi: 10.1155/2018/4596141. eCollection 2018. J Anal Methods Chem. 2018. PMID: 30275997 Free PMC article.
Cited by
-
Determination of flutamide and two major metabolites using HPLC-DAD and HPTLC methods.Chem Cent J. 2018 Jan 25;12(1):4. doi: 10.1186/s13065-018-0372-y. Chem Cent J. 2018. PMID: 29372342 Free PMC article.
-
Novel RP-HPLC-DAD approach for simultaneous determination of chlorphenoxamine hydrochloride and caffeine with their related substances.BMC Chem. 2024 Jul 19;18(1):133. doi: 10.1186/s13065-024-01238-8. BMC Chem. 2024. PMID: 39030644 Free PMC article.
-
Characterization of the Effect of Drug-Drug Interaction on Protein Binding in Concurrent Administration of Sulfamethoxazol and Diclofenac Sodium Using Bovine Serum Albumin.Adv Pharm Bull. 2016 Dec;6(4):589-595. doi: 10.15171/apb.2016.073. Epub 2016 Dec 22. Adv Pharm Bull. 2016. PMID: 28101466 Free PMC article.
-
Synergistic innovation: MOF@GCN hybrid for electrochemical detection of flutamide-bridging experimental, computational, and real-world applications.Mikrochim Acta. 2025 Apr 16;192(5):303. doi: 10.1007/s00604-025-07164-3. Mikrochim Acta. 2025. PMID: 40240687
-
Application of 19F NMR Spectroscopy for Content Determination of Fluorinated Pharmaceuticals.J Anal Methods Chem. 2017;2017:9206297. doi: 10.1155/2017/9206297. Epub 2017 Oct 18. J Anal Methods Chem. 2017. PMID: 29181224 Free PMC article.
References
-
- Álvarez-Lueje A, Peña C, Núñez-Vergara LJ, Squella JA. Electrochemical Study of Flutamide, an Anticancer Drug, and Its Polarographic, UV Spectrophotometric and HPLC Determination in Tablets. Electroanal. 1998;10(15):1043–51.
-
- Shet MS, McPhaul M, Fisher CW, Stallings NR, Estabrook RW. Metabolism of the antiandrogenic drug (Flutamide) by human CYP1A2. Drug Metab Dispos. 1997;25(11):1298–303. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases