Thrombin Cleavage of Osteopontin Modulates Its Activities in Human Cells In Vitro and Mouse Experimental Autoimmune Encephalomyelitis In Vivo
- PMID: 27478856
- PMCID: PMC4961817
- DOI: 10.1155/2016/9345495
Thrombin Cleavage of Osteopontin Modulates Its Activities in Human Cells In Vitro and Mouse Experimental Autoimmune Encephalomyelitis In Vivo
Abstract
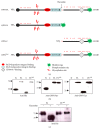
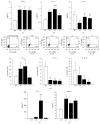
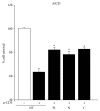
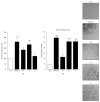
Osteopontin is a proinflammatory cytokine and plays a pathogenetic role in multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis (EAE), by recruiting autoreactive T cells into the central nervous system. Osteopontin functions are modulated by thrombin cleavage generating N- and C-terminal fragment, whose individual roles are only partly known. Published data are difficult to compare since they have been obtained with heterogeneous approaches. Interestingly, thrombin cleavage of osteopontin unmasks a cryptic domain of interaction with α 4 β 1 integrin that is the main adhesion molecule involved in lymphocyte transmigration to the brain and is the target for natalizumab, the most potent drug preventing relapses. We produced recombinant osteopontin and its N- and C-terminal fragments in an eukaryotic system in order to allow their posttranslational modifications. We investigated, in vitro, their effect on human cells and in vivo in EAE. We found that the osteopontin cleavage plays a key role in the function of this cytokine and that the two fragments exert distinct effects both in vitro and in vivo. These findings suggest that drugs targeting each fragment may be used to fine-tune the pathological effects of osteopontin in several diseases.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

