Co-Registration of Bioluminescence Tomography, Computed Tomography, and Magnetic Resonance Imaging for Multimodal In Vivo Stem Cell Tracking
- PMID: 27478872
- PMCID: PMC4966683
- DOI: 10.18383/j.tom.2016.00160
Co-Registration of Bioluminescence Tomography, Computed Tomography, and Magnetic Resonance Imaging for Multimodal In Vivo Stem Cell Tracking
Abstract
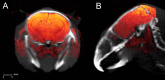
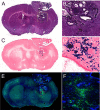
We present a practical approach for co-registration of bioluminescence tomography (BLT), computed tomography (CT), and magnetic resonance (MR) images. To this end, we developed a customized animal shuttle composed of non-fluorescent, MR-compatible Delrin plastic that fits a commercially available MR surface coil. Mouse embryonic stem cells (mESCs) were transfected with the luciferase gene and labeled with superparamagnetic iron oxide (SPIO) nanoparticles. Cells were stereotaxically implanted in mouse brain and imaged weekly for 4 weeks with BLI (IVIS Spectrum CT scanner) and MRI (11.7T horizontal bore scanner). Without the use of software co-registration, in vitro phantom studies yielded root-mean-square errors (RMSE) of 7.6×10-3, 0.93 mm, and 0.78 mm along the medial-lateral (ML), dorsal-ventral (DV), and anterior-posterior (AP) axes, respectively. Rotation errors were negligible. Software co-registration by translation along the DV and AP axes resulted in consistent agreement between the CT and MR images, without the need for rotation or warping. In vivo co-registered BLT/MRI mouse brain data sets demonstrated a single, diffuse region of BLI photon signal and MRI hypointensity. Over time, the transplanted cells formed tumors as validated by histopathology. Disagreement between BLT and MRI tumor location was greatest along the DV axis (1.4±0.2 mm) compared to the ML (0.5±0.3 mm) and AP axis (0.6 mm) due to the uncertainty of the depth of origin of the BLT signal. Combining the high spatial anatomical information of MRI with the cell viability/proliferation data from BLT should facilitate pre-clinical evaluation of novel therapeutic candidate stem cells.
Keywords: Cell tracking; Computed tomography; Multimodal imaging; Stem cells; bioluminescence imaging; co-registration; magnetic resonance imaging.
Conflict of interest statement
Conflict of Interest: The authors have no disclosures to report.
Figures





Similar articles
-
In vivo visualization and ex vivo quantification of murine breast cancer cells in the mouse brain using MRI cell tracking and electron paramagnetic resonance.NMR Biomed. 2015 Mar;28(3):367-75. doi: 10.1002/nbm.3259. Epub 2015 Jan 22. NMR Biomed. 2015. PMID: 25611487
-
SU-E-J-198: Bioluminescence Monitoring of Metastatic Breast Cancer: Quantitative Assessment of Radiation Treatment Effects and Tracking of Tumor Cells.Med Phys. 2012 Jun;39(6Part9):3698. doi: 10.1118/1.4735039. Med Phys. 2012. PMID: 28519066
-
Multimodal imaging of subventricular zone neural stem/progenitor cells in the cuprizone mouse model reveals increased neurogenic potential for the olfactory bulb pathway, but no contribution to remyelination of the corpus callosum.Neuroimage. 2014 Feb 1;86:99-110. doi: 10.1016/j.neuroimage.2013.07.080. Epub 2013 Aug 7. Neuroimage. 2014. PMID: 23933305
-
In vivo tracking of stem cells in brain and spinal cord injury.Prog Brain Res. 2007;161:367-83. doi: 10.1016/S0079-6123(06)61026-1. Prog Brain Res. 2007. PMID: 17618991 Review.
-
Fluorescence-Based Mono- and Multimodal Imaging for In Vivo Tracking of Mesenchymal Stem Cells.Biomolecules. 2023 Dec 13;13(12):1787. doi: 10.3390/biom13121787. Biomolecules. 2023. PMID: 38136656 Free PMC article. Review.
Cited by
-
Multimodal Imaging-Guided Stem Cell Ocular Treatment.ACS Nano. 2024 Jun 11;18(23):14893-14906. doi: 10.1021/acsnano.3c10632. Epub 2024 May 27. ACS Nano. 2024. PMID: 38801653 Free PMC article.
-
In Vivo Subretinal ARPE-19 Cell Tracking Using Indocyanine Green Contrast-Enhanced Multimodality Photoacoustic Microscopy, Optical Coherence Tomography, and Fluorescence Imaging for Regenerative Medicine.Transl Vis Sci Technol. 2021 Aug 12;10(10):10. doi: 10.1167/tvst.10.10.10. Transl Vis Sci Technol. 2021. PMID: 34473239 Free PMC article.
-
Nanotechnology-Assisted Cell Tracking.Nanomaterials (Basel). 2022 Apr 20;12(9):1414. doi: 10.3390/nano12091414. Nanomaterials (Basel). 2022. PMID: 35564123 Free PMC article. Review.
-
Options for imaging cellular therapeutics in vivo: a multi-stakeholder perspective.Cytotherapy. 2021 Sep;23(9):757-773. doi: 10.1016/j.jcyt.2021.02.005. Epub 2021 Apr 6. Cytotherapy. 2021. PMID: 33832818 Free PMC article. Review.
-
A review of the application of machine learning in molecular imaging.Ann Transl Med. 2021 May;9(9):825. doi: 10.21037/atm-20-5877. Ann Transl Med. 2021. PMID: 34268438 Free PMC article. Review.
References
-
- Contag CH, Ross BD. It's not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging. 2002;16(4):378–387. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
