Differentiation stage of myeloma plasma cells: biological and clinical significance
- PMID: 27479184
- PMCID: PMC5439510
- DOI: 10.1038/leu.2016.211
Differentiation stage of myeloma plasma cells: biological and clinical significance
Abstract
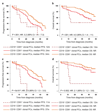
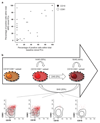
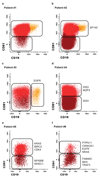
The notion that plasma cells (PCs) are terminally differentiated has prevented intensive research in multiple myeloma (MM) about their phenotypic plasticity and differentiation. Here, we demonstrated in healthy individuals (n=20) that the CD19-CD81 expression axis identifies three bone marrow (BM)PC subsets with distinct age-prevalence, proliferation, replication-history, immunoglobulin-production, and phenotype, consistent with progressively increased differentiation from CD19+CD81+ into CD19-CD81+ and CD19-CD81- BMPCs. Afterwards, we demonstrated in 225 newly diagnosed MM patients that, comparing to normal BMPC counterparts, 59% had fully differentiated (CD19-CD81-) clones, 38% intermediate-differentiated (CD19-CD81+) and 3% less-differentiated (CD19+CD81+) clones. The latter patients had dismal outcome, and PC differentiation emerged as an independent prognostic marker for progression-free (HR: 1.7; P=0.005) and overall survival (HR: 2.1; P=0.006). Longitudinal comparison of diagnostic vs minimal-residual-disease samples (n=40) unraveled that in 20% of patients, less-differentiated PCs subclones become enriched after therapy-induced pressure. We also revealed that CD81 expression is epigenetically regulated, that less-differentiated clonal PCs retain high expression of genes related to preceding B-cell stages (for example: PAX5), and show distinct mutation profile vs fully differentiated PC clones within individual patients. Together, we shed new light into PC plasticity and demonstrated that MM patients harbouring less-differentiated PCs have dismal survival, which might be related to higher chemoresistant potential plus different molecular and genomic profiles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701–4705. - PubMed
-
- Flores-Montero J, de Tute R, Paiva B, Perez JJ, Bottcher S, Wind H, et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry B Clin Cytom. 2015;90:61–72. - PubMed
-
- Mateo G, Montalban MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutierrez N, et al. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. J Clin Oncol. 2008;26:2737–2744. - PubMed
-
- Paiva B, Gutierrez NC, Chen X, Vidriales MB, Montalban MA, Rosinol L, et al. Clinical significance of CD81 expression by clonal plasma cells in high-risk smoldering and symptomatic multiple myeloma patients. Leukemia. 2012;26:1862–1869. - PubMed
-
- Bataille R, Jego G, Robillard N, Barille-Nion S, Harousseau JL, Moreau P, et al. The phenotype of normal, reactive and malignant plasma cells. Identification of ‘many and multiple myelomas’ and of new targets for myeloma therapy. Haematologica. 2006;91:1234–1240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

