Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation
- PMID: 27479603
- PMCID: PMC5007193
- DOI: 10.1038/ncb3394
Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation
Abstract
Bone-marrow-derived skeletal stem/stromal cell (SSC) self-renewal and function are critical to skeletal development, homeostasis and repair. Nevertheless, the mechanisms controlling SSC behaviour, particularly bone formation, remain ill-defined. Using knockout mouse models that target the zinc-finger transcription factors Snail or Slug, or Snail and Slug combined, a regulatory axis has been uncovered wherein Snail and Slug cooperatively control SSC self-renewal, osteoblastogenesis and bone formation. Mechanistically, Snail/Slug regulate SSC function by forming complexes with the transcriptional co-activators YAP and TAZ in tandem with the inhibition of the Hippo-pathway-dependent regulation of YAP/TAZ signalling cascades. In turn, the Snail/Slug-YAP/TAZ axis activates a series of YAP/TAZ/TEAD and Runx2 downstream targets that control SSC homeostasis and osteogenesis. Together, these results demonstrate that SSCs mobilize Snail/Slug-YAP/TAZ complexes to control stem cell function.
Figures

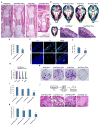



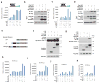


References
-
- Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16:239–253. - PubMed
-
- Barrallo-Gimeno A, Nieto MA. Evolutionary history of the Snail/Scratch superfamily. Trends Genet. 2009;25:248–252. - PubMed
-
- Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342:1234850. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

