TGFβ regulates persistent neuroinflammation by controlling Th1 polarization and ROS production via monocyte-derived dendritic cells
- PMID: 27479807
- PMCID: PMC5053226
- DOI: 10.1002/glia.23033
TGFβ regulates persistent neuroinflammation by controlling Th1 polarization and ROS production via monocyte-derived dendritic cells
Abstract
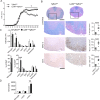
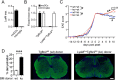
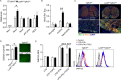
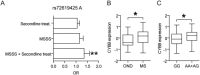
Intracerebral levels of Transforming Growth Factor beta (TGFβ) rise rapidly during the onset of experimental autoimmune encephalomyelitis (EAE), a mouse model of Multiple Sclerosis (MS). We addressed the role of TGFβ responsiveness in EAE by targeting the TGFβ receptor in myeloid cells, determining that Tgfbr2 was specifically targeted in monocyte-derived dendritic cells (moDCs) but not in CNS resident microglia by using bone-marrow chimeric mice. TGFβ responsiveness in moDCs was necessary for the remission phase since LysM(Cre) Tgfbr2(fl/fl) mice developed a chronic form of EAE characterized by severe demyelination and extensive infiltration of activated moDCs in the CNS. Tgfbr2 deficiency resulted in increased moDC IL-12 secretion that skewed T cells to produce IFN-γ, which in turn enhanced the production of moDC-derived reactive oxygen species that promote oxidative damage and demyelination. We identified SNPs in the human NOX2 (CYBB) gene that associated with the severity of MS, and significantly increased CYBB expression was recorded in PBMCs from both MS patients and from MS severity risk allele rs72619425-A carrying individuals. We thus identify a novel myeloid cell-T cell activation loop active in the CNS during chronic disease that could be therapeutically targeted. GLIA 2016;64:1925-1937.
Keywords: MOG-EAE; TGFβ; gene deletion; monocyte-derived dendritic cells; reactive oxygen species.
© 2016 The Authors. Glia Published by Wiley Periodicals, Inc.
Figures


References
-
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FMV. 2011. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 14:1142–1149. - PubMed
-
- Biollaz G, Bernasconi L, Cretton C, Püntener U, Frei K, Fontana A, Suter T. 2009. Site‐specific anti‐tumor immunity: Differences in DC function, TGF‐β production and numbers of intratumoral Foxp3+ Treg. Eur J Immunol 39:1323–1333. - PubMed
-
- Codarri L, Greter M, Becher B. 2013. Communication between pathogenic T cells and myeloid cells in neuroinflammatory disease. Trends Immunol 34:114–119. - PubMed
-
- Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. 2011. RORγt drives production of the cytokine GM‐CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12:560–567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

