Probiotics: Prevention of Severe Pneumonia and Endotracheal Colonization Trial-PROSPECT: a pilot trial
- PMID: 27480757
- PMCID: PMC4970233
- DOI: 10.1186/s13063-016-1495-x
Probiotics: Prevention of Severe Pneumonia and Endotracheal Colonization Trial-PROSPECT: a pilot trial
Abstract
Background: Probiotics are live microorganisms that may confer health benefits when ingested. Randomized trials suggest that probiotics significantly decrease the incidence of ventilator-associated pneumonia (VAP) and the overall incidence of infection in critically ill patients. However, these studies are small, largely single-center, and at risk of bias. The aim of the PROSPECT pilot trial was to determine the feasibility of conducting a larger trial of probiotics to prevent VAP in mechanically ventilated patients in the intensive care unit (ICU).
Methods: In a randomized blinded trial, patients expected to be mechanically ventilated for ≥72 hours were allocated to receive either 1 × 10(10) colony-forming units of Lactobacillus rhamnosus GG or placebo, twice daily. Patients were excluded if they were at increased risk of L. rhamnosus GG infection or had contraindications to enteral medication. Feasibility objectives were: (1) timely recruitment; (2) maximal protocol adherence; (3) minimal contamination; and (4) estimated VAP rate ≥10 %. We also measured other infections, diarrhea, ICU and hospital length of stay, and mortality.
Results: Overall, in 14 centers in Canada and the USA, all feasibility goals were met: (1) 150 patients were randomized in 1 year; (2) protocol adherence was 97 %; (3) no patients received open-label probiotics; and (4) the VAP rate was 19 %. Other infections included: bloodstream infection (19.3 %), urinary tract infections (12.7 %), and skin and soft tissue infections (4.0 %). Diarrhea, defined as Bristol type 6 or 7 stools, occurred in 133 (88.7 %) of patients, the median length of stay in ICU was 12 days (quartile 1 to quartile 3, 7-18 days), and in hospital was 26 days (quartile 1 to quartile 3, 14-44 days); 23 patients (15.3 %) died in the ICU.
Conclusions: The PROSPECT pilot trial supports the feasibility of a larger trial to investigate the effect of L. rhamnosus GG on VAP and other nosocomial infections in critically ill patients.
Trial registration: Clinicaltrials.gov NCT01782755 . Registered on 29 January 2013.
Keywords: Critically ill; Infection; Intensive care; Probiotics.
Figures
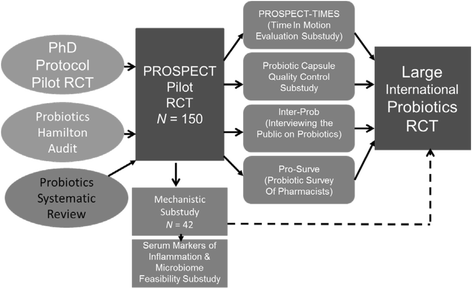
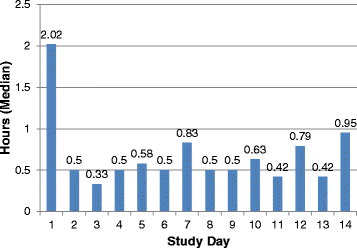
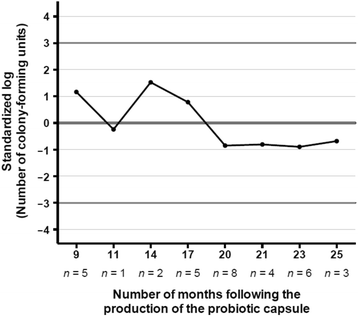
References
-
- Joint FAO/WHO Working Group. Guidelines for the evaluation of probiotics in food: report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed 26 Dec 2014.
-
- Hao Q, Lu Z, Dong BR, Huang CQ, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2011;9:CD006895. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

