The differential short- and long-term effects of HIV-1 latency-reversing agents on T cell function
- PMID: 27480951
- PMCID: PMC4969750
- DOI: 10.1038/srep30749
The differential short- and long-term effects of HIV-1 latency-reversing agents on T cell function
Erratum in
-
Corrigendum: The differential short- and long-term effects of HIV-1 latency-reversing agents on T cell function.Sci Rep. 2016 Oct 3;6:34430. doi: 10.1038/srep34430. Sci Rep. 2016. PMID: 27694881 Free PMC article. No abstract available.
Abstract
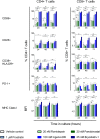
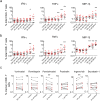
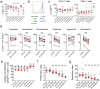
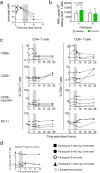
Despite the extraordinary success of HIV-1 antiretroviral therapy in prolonging life, infected individuals face lifelong therapy because of a reservoir of latently-infected cells that harbor replication competent virus. Recently, compounds have been identified that can reverse HIV-1 latency in vivo. These latency- reversing agents (LRAs) could make latently-infected cells vulnerable to clearance by immune cells, including cytolytic CD8+ T cells. We investigated the effects of two leading LRA classes on CD8+ T cell phenotype and function: the histone deacetylase inhibitors (HDACis) and protein kinase C modulators (PKCms). We observed that relative to HDACis, the PKCms induced much stronger T cell activation coupled with non-specific cytokine production and T cell proliferation. When examining antigen-specific CD8+ T cell function, all the LRAs except the HDACi Vorinostat reduced, but did not abolish, one or more measurements of CD8+ T cell function. Importantly, the extent and timing of these effects differed between LRAs. Panobinostat had detrimental effects within 10 hours of drug treatment, whereas the effects of the other LRAs were observed between 48 hours and 5 days. These observations suggest that scheduling of LRA and CD8+ T cell immunotherapy regimens may be critical for optimal clearance of the HIV-1 reservoir.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

