GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis
- PMID: 27481132
- PMCID: PMC4995082
- DOI: 10.1084/jem.20160061
GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis
Abstract
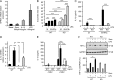
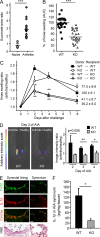
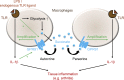
When SUCNR1/GPR91-expressing macrophages are activated by inflammatory signals, they change their metabolism and accumulate succinate. In this study, we show that during this activation, macrophages release succinate into the extracellular milieu. They simultaneously up-regulate GPR91, which functions as an autocrine and paracrine sensor for extracellular succinate to enhance IL-1β production. GPR91-deficient mice lack this metabolic sensor and show reduced macrophage activation and production of IL-1β during antigen-induced arthritis. Succinate is abundant in synovial fluids from rheumatoid arthritis (RA) patients, and these fluids elicit IL-1β release from macrophages in a GPR91-dependent manner. Together, we reveal a GPR91/succinate-dependent feed-forward loop of macrophage activation and propose GPR91 antagonists as novel therapeutic principles to treat RA.
© 2016 Littlewood-Evans et al.
Figures

References
-
- Bresnihan B., Pontifex E., Thurlings R.M., Vinkenoog M., El-Gabalawy H., Fearon U., Fitzgerald O., Gerlag D.M., Rooney T., van de Sande M.G., et al. . 2009. Synovial tissue sublining CD68 expression is a biomarker of therapeutic response in rheumatoid arthritis clinical trials: consistency across centers. J. Rheumatol. 36:1800–1802. 10.3899/jrheum.090348 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

