Phage Therapy in the Era of Synthetic Biology
- PMID: 27481531
- PMCID: PMC5046696
- DOI: 10.1101/cshperspect.a023879
Phage Therapy in the Era of Synthetic Biology
Abstract
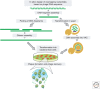
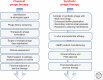
For more than a century, bacteriophage (or phage) research has enabled some of the most important discoveries in biological sciences and has equipped scientists with many of the molecular biology tools that have advanced our understanding of replication, maintenance, and expression of genetic material. Phages have also been recognized and exploited as natural antimicrobial agents and nanovectors for gene therapy, but their potential as therapeutics has not been fully exploited in Western medicine because of challenges such as narrow host range, bacterial resistance, and unique pharmacokinetics. However, increasing concern related to the emergence of bacteria resistant to multiple antibiotics has heightened interest in phage therapy and the development of strategies to overcome hurdles associated with bacteriophage therapeutics. Recent progress in sequencing technologies, DNA manipulation, and synthetic biology allowed scientists to refactor the entire bacterial genome of Mycoplasma mycoides, thereby creating the first synthetic cell. These new strategies for engineering genomes may have the potential to accelerate the construction of designer phage genomes with superior therapeutic potential. Here, we discuss the use of phage as therapeutics, as well as how synthetic biology can create bacteriophage with desirable attributes.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Abedon S. 2011. Phage therapy pharmacology: Calculating phage dosing. Adv Appl Microbiol 77: 1–40. - PubMed
-
- Alisky J, Iczkowski K, Rapoport A, Troitsky N. 1998. Bacteriophages show promise as antimicrobial agents. J Infect 36: 5–15. - PubMed
-
- Arias CA, Murray BE. 2009. Antibiotic-resistant bugs in the 21st century—A clinical super-challenge. N Engl J Med 360: 439–443. - PubMed
-
- Barrett ADT, Stanberry LR. 2008. Vaccines for biodefence and emerging and neglected diseases. Academic, Waltham, MA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
