Second Messengers
- PMID: 27481708
- PMCID: PMC4968160
- DOI: 10.1101/cshperspect.a005926
Second Messengers
Abstract
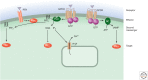
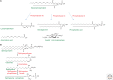
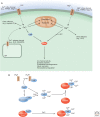
Second messengers are small molecules and ions that relay signals received by cell-surface receptors to effector proteins. They include a wide variety of chemical species and have diverse properties that allow them to signal within membranes (e.g., hydrophobic molecules such as lipids and lipid derivatives), within the cytosol (e.g., polar molecules such as nucleotides and ions), or between the two (e.g., gases and free radicals). Second messengers are typically present at low concentrations in resting cells and can be rapidly produced or released when cells are stimulated. The levels of second messengers are exquisitely controlled temporally and spatially, and, during signaling, enzymatic reactions or opening of ion channels ensure that they are highly amplified. These messengers then diffuse rapidly from the source and bind to target proteins to alter their properties (activity, localization, stability, etc.) to propagate signaling.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Baumgartner HK, Gerasimenko JV, Thorne C, Ferdek P, Pozzan T, Tepikin AV, Petersen OH, Sutton R, Watson AJ, Gerasimenko OV. 2009. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J Biol Chem 284: 20796–20803. - PMC - PubMed
-
- Beavo JA, Brunton LL. 2002. Cyclic nucleotide research—Still expanding after half a century. Nat Rev Mol Cell Biol 3: 710–718. - PubMed
-
- Berridge MJ. 2004. Calcium signal transduction and cellular control mechanisms. Biochim Biophys Acta 1742: 3–7. - PubMed
-
- Berridge MJ. 2006. Calcium microdomains: Organization and function. Cell Calcium 40: 405–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
