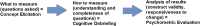Patient-Reported Outcome Assessments as Endpoints in Studies in Infectious Diseases
- PMID: 27481954
- PMCID: PMC5006216
- DOI: 10.1093/cid/ciw317
Patient-Reported Outcome Assessments as Endpoints in Studies in Infectious Diseases
Abstract
The goal of administering medical interventions is to help patients live longer or live better. In keeping with this goal, there has been increasing interest in taking the "voice" of the patient into account during the development process, specifically in the evaluation of treatment benefits of medical interventions, and use of patient-centered outcome data to justify reimbursement. Patient-reported outcomes (PROs) are outcome assessments (OAs) used to define endpoints that can provide direct evidence of treatment benefit on how patients feel or function. When PROs are appropriately developed, they can increase the efficiency and clinical relevance of clinical trials. Several PROs have been developed for OA in specific infectious diseases indications, and more are under development. PROs also hold promise for use in evaluating adherence, adverse effects, satisfaction with care, and routine clinical practice.
Keywords: clinical practice; clinical trials; endpoints; infectious diseases; patient-reported outcomes.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures
References
-
- Patrick DL, Burke LB, Powers JH et al. . Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health 2007; 10(suppl 2):S125–37. - PubMed
-
- Chassany O, Sagnier P, Marquis P, Fullerton S, Aaronson N. Patient-reported outcomes: the example of health-related quality of life—a European guidance document for the improved integration of health-related quality of life assessment in the drug regulatory process. Drug Inf J 2002; 36:209–38.
-
- Washington AE, Lipstein SH. The Patient-Centered Outcomes Research Institute—promoting better information, decisions, and health. N Engl J Med 2011; 365:e31. - PubMed
-
- Feeny D, Eckstrom E, Whitlock EP, Perdue LA. Agency for Healthcare Research and Quality: a primer for systematic reviewers on the measurement of functional status and health-related quality of life in older adults, 2013. Available at: http://www.ncbi.nlm.nih.gov/books/NBK169159/pdf/Bookshelf_NBK169159.pdf. Accessed 25 May 2016. - PubMed
-
- Gabriel SE, Normand SL. Getting the methods right—the foundation of patient-centered outcomes research. N Engl J Med 2012; 367:787–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous