BLOC-1 and BLOC-3 regulate VAMP7 cycling to and from melanosomes via distinct tubular transport carriers
- PMID: 27482051
- PMCID: PMC4970331
- DOI: 10.1083/jcb.201605090
BLOC-1 and BLOC-3 regulate VAMP7 cycling to and from melanosomes via distinct tubular transport carriers
Abstract
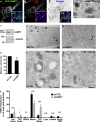
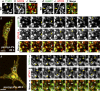
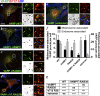
Endomembrane organelle maturation requires cargo delivery via fusion with membrane transport intermediates and recycling of fusion factors to their sites of origin. Melanosomes and other lysosome-related organelles obtain cargoes from early endosomes, but the fusion machinery involved and its recycling pathway are unknown. Here, we show that the v-SNARE VAMP7 mediates fusion of melanosomes with tubular transport carriers that also carry the cargo protein TYRP1 and that require BLOC-1 for their formation. Using live-cell imaging, we identify a pathway for VAMP7 recycling from melanosomes that employs distinct tubular carriers. The recycling carriers also harbor the VAMP7-binding scaffold protein VARP and the tissue-restricted Rab GTPase RAB38. Recycling carrier formation is dependent on the RAB38 exchange factor BLOC-3. Our data suggest that VAMP7 mediates fusion of BLOC-1-dependent transport carriers with melanosomes, illuminate SNARE recycling from melanosomes as a critical BLOC-3-dependent step, and likely explain the distinct hypopigmentation phenotypes associated with BLOC-1 and BLOC-3 deficiency in Hermansky-Pudlak syndrome variants.
© 2016 Dennis et al.
Figures







Comment in
-
Reduce, reuse, recycle: a retrieval transport pathway for the membrane fusion machinery involved in melanosome biogenesis.Pigment Cell Melanoma Res. 2017 Jan;30(1):10-12. doi: 10.1111/pcmr.12551. Pigment Cell Melanoma Res. 2017. PMID: 27804227 No abstract available.
References
-
- Anderson P.D., Huizing M., Claassen D.A., White J., and Gahl W.A.. 2003. Hermansky-Pudlak syndrome type 4 (HPS-4): clinical and molecular characteristics. Hum. Genet. 113:10–17. - PubMed
-
- Boissy R.E., Zhao Y., and Gahl W.A.. 1998. Altered protein localization in melanocytes from Hermansky-Pudlak syndrome: support for the role of the HPS gene product in intracellular trafficking. Lab. Invest. 78:1037–1048. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

