Assembly and activation of dynein-dynactin by the cargo adaptor protein Hook3
- PMID: 27482052
- PMCID: PMC4970328
- DOI: 10.1083/jcb.201604002
Assembly and activation of dynein-dynactin by the cargo adaptor protein Hook3
Abstract
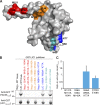
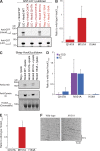
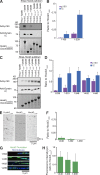
Metazoan cytoplasmic dynein moves processively along microtubules with the aid of dynactin and an adaptor protein that joins dynein and dynactin into a stable ternary complex. Here, we examined how Hook3, a cargo adaptor involved in Golgi and endosome transport, forms a motile dynein-dynactin complex. We show that the conserved Hook domain interacts directly with the dynein light intermediate chain 1 (LIC1). By solving the crystal structure of the Hook domain and using structure-based mutagenesis, we identify two conserved surface residues that are each critical for LIC1 binding. Hook proteins with mutations in these residues fail to form a stable dynein-dynactin complex, revealing a crucial role for LIC1 in this interaction. We also identify a region of Hook3 specifically required for an allosteric activation of processive motility. Our work reveals the structural details of Hook3's interaction with dynein and offers insight into how cargo adaptors form processive dynein-dynactin motor complexes.
© 2016 Schroeder and Vale.
Figures

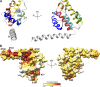
References
-
- Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.-W., Kapral G.J., Grosse-Kunstleve R.W., et al. . 2010. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

