Dynamic periplasmic chaperone reservoir facilitates biogenesis of outer membrane proteins
- PMID: 27482090
- PMCID: PMC4995976
- DOI: 10.1073/pnas.1601002113
Dynamic periplasmic chaperone reservoir facilitates biogenesis of outer membrane proteins
Abstract
Outer membrane protein (OMP) biogenesis is critical to bacterial physiology because the cellular envelope is vital to bacterial pathogenesis and antibiotic resistance. The process of OMP biogenesis has been studied in vivo, and each of its components has been studied in isolation in vitro. This work integrates parameters and observations from both in vivo and in vitro experiments into a holistic computational model termed "Outer Membrane Protein Biogenesis Model" (OMPBioM). We use OMPBioM to assess OMP biogenesis mathematically in a global manner. Using deterministic and stochastic methods, we are able to simulate OMP biogenesis under varying genetic conditions, each of which successfully replicates experimental observations. We observe that OMPs have a prolonged lifetime in the periplasm where an unfolded OMP makes, on average, hundreds of short-lived interactions with chaperones before folding into its native state. We find that some periplasmic chaperones function primarily as quality-control factors; this function complements the folding catalysis function of other chaperones. Additionally, the effective rate for the β-barrel assembly machinery complex necessary for physiological folding was found to be higher than has currently been observed in vitro. Overall, we find a finely tuned balance between thermodynamic and kinetic parameters maximizes OMP folding flux and minimizes aggregation and unnecessary degradation. In sum, OMPBioM provides a global view of OMP biogenesis that yields unique insights into this essential pathway.
Keywords: BAM; OMP biogenesis; kinetic model; membrane protein folding; β-barrel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
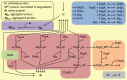
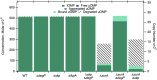
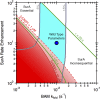
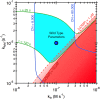
References
-
- Tamm LK, Hong H, Liang B. Folding and assembly of β-barrel membrane proteins. Biochim Biophys Acta - Biomembr. 2004;1666(1-2):250–263. - PubMed
-
- Wimley WC. The versatile β-barrel membrane protein. Curr Opin Struct Biol. 2003;13(4):404–411. - PubMed
-
- Bajaj H, et al. Antibiotic uptake through membrane channels: Role of Providencia stuartii OmpPst1 porin in carbapenem resistance. Biochemistry. 2012;51(51):10244–10249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

