CCN1/CYR61-mediated meticulous patrolling by Ly6Clow monocytes fuels vascular inflammation
- PMID: 27482114
- PMCID: PMC4995973
- DOI: 10.1073/pnas.1607710113
CCN1/CYR61-mediated meticulous patrolling by Ly6Clow monocytes fuels vascular inflammation
Erratum in
-
Correction for Imhof et al., CCN1/CYR61-mediated meticulous patrolling by Ly6Clow monocytes fuels vascular inflammation.Proc Natl Acad Sci U S A. 2019 Mar 26;116(13):6501. doi: 10.1073/pnas.1903306116. Epub 2019 Mar 18. Proc Natl Acad Sci U S A. 2019. PMID: 30886099 Free PMC article. No abstract available.
Abstract
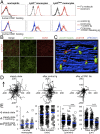
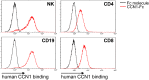
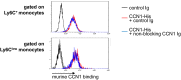
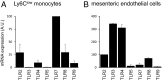
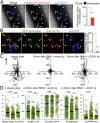
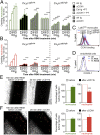
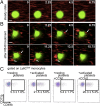
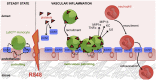
Inflammation is characterized by the recruitment of leukocytes from the bloodstream. The rapid arrival of neutrophils is followed by a wave of inflammatory lymphocyte antigen 6 complex (Ly6C)-positive monocytes. In contrast Ly6C(low) monocytes survey the endothelium in the steady state, but their role in inflammation is still unclear. Here, using confocal intravital microscopy, we show that upon Toll-like receptor 7/8 (TLR7/8)-mediated inflammation of mesenteric veins, platelet activation drives the rapid mobilization of Ly6C(low) monocytes to the luminal side of the endothelium. After repeatedly interacting with platelets, Ly6C(low) monocytes commit to a meticulous patrolling of the endothelial wall and orchestrate the subsequent arrival and extravasation of neutrophils through the production of proinflammatory cytokines and chemokines. At a molecular level, we show that cysteine-rich protein 61 (CYR61)/CYR61 connective tissue growth factor nephroblastoma overexpressed 1 (CCN1) protein is released by activated platelets and enables the recruitment of Ly6C(low) monocytes upon vascular inflammation. In addition endothelium-bound CCN1 sustains the adequate patrolling of Ly6C(low) monocytes both in the steady state and under inflammatory conditions. Blocking CCN1 or platelets with specific antibodies impaired the early arrival of Ly6C(low) monocytes and abolished the recruitment of neutrophils. These results refine the leukocyte recruitment cascade model by introducing endothelium-bound CCN1 as an inflammation mediator and by demonstrating a role for platelets and patrolling Ly6C(low) monocytes in acute vascular inflammation.
Keywords: CCN1; inflammation; monocyte; neutrophil; platelet.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






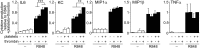
References
-
- Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114(21):4613–4623. - PubMed
-
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. - PubMed
-
- Sunderkötter C, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172(7):4410–4417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

