Dorsal Medial Habenula Regulation of Mood-Related Behaviors and Primary Reinforcement by Tachykinin-Expressing Habenula Neurons
- PMID: 27482535
- PMCID: PMC4947983
- DOI: 10.1523/ENEURO.0109-16.2016
Dorsal Medial Habenula Regulation of Mood-Related Behaviors and Primary Reinforcement by Tachykinin-Expressing Habenula Neurons
Abstract
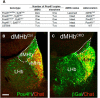
Animal models have been developed to investigate aspects of stress, anxiety, and depression, but our understanding of the circuitry underlying these models remains incomplete. Prior studies of the habenula, a poorly understood nucleus in the dorsal diencephalon, suggest that projections to the medial habenula (MHb) regulate fear and anxiety responses, whereas the lateral habenula (LHb) is involved in the expression of learned helplessness, a model of depression. Tissue-specific deletion of the transcription factor Pou4f1 in the dorsal MHb (dMHb) results in a developmental lesion of this subnucleus. These dMHb-ablated mice show deficits in voluntary exercise, a possible correlate of depression. Here we explore the role of the dMHb in mood-related behaviors and intrinsic reinforcement. Lesions of the dMHb do not elicit changes in contextual conditioned fear. However, dMHb-lesioned mice exhibit shorter immobility time in the tail suspension test, another model of depression. dMHb-lesioned mice also display increased vulnerability to the induction of learned helplessness. However, this effect is not due specifically to the dMHb lesion, but appears to result from Pou4f1 haploinsufficiency elsewhere in the nervous system. Pou4f1 haploinsufficiency does not produce the other phenotypes associated with dMHb lesions. Using optogenetic intracranial self-stimulation, intrinsic reinforcement by the dMHb can be mapped to a specific population of neurokinin-expressing habenula neurons. Together, our data show that the dMHb is involved in the regulation of multiple mood-related behaviors, but also support the idea that these behaviors do not reflect a single functional pathway.
Keywords: fear conditioning; habenula; interpeduncular nucleus; learned helplessness; substance P.
Conflict of interest statement
Figures







References
-
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF (2001) The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res 917:118–126. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
