Recognition of influenza H3N2 variant virus by human neutralizing antibodies
- PMID: 27482543
- PMCID: PMC4962875
- DOI: 10.1172/jci.insight.86673
Recognition of influenza H3N2 variant virus by human neutralizing antibodies
Abstract
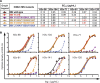
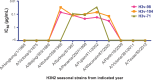
Since 2011, over 300 human cases of infection, especially in exposed children, with the influenza A H3N2 variant (H3N2v) virus that circulates in swine in the US have been reported. The structural and genetic basis for the lack of protection against H3N2v induced by vaccines containing seasonal H3N2 antigens is poorly understood. We isolated 17 human monoclonal antibodies (mAbs) that neutralized H3N2v virus from subjects experimentally immunized with an H3N2v candidate vaccine. Six mAbs exhibited very potent neutralizing activity (IC50 < 200 ng/ml) against the H3N2v virus but not against current human H3N2 circulating strains. Fine epitope mapping and structural characterization of antigen-antibody complexes revealed that H3N2v specificity was attributable to amino acid polymorphisms in the 150-loop and the 190-helix antigenic sites on the hemagglutinin protein. H3N2v-specific antibodies also neutralized human H3N2 influenza strains naturally circulating between 1995 and 2005. These results reveal a high level of antigenic relatedness between the swine H3N2v virus and previously circulating human strains, consistent with the fact that early human H3 seasonal strains entered the porcine population in the 1990s and reentered the human population, where they had not been circulating, as H3N2v about a decade later. The data also explain the increased susceptibility to H3N2v viruses in young children, who lack prior exposure to human seasonal strains from the 1990s.
Figures




References
-
- World Health Organization Influenza (Seasonal) [June 13, 2016];WHO Web site. http://www.who.int/mediacentre/factsheets/fs211/en/
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

