The mitogen and stress-activated protein kinase 1 regulates the rapid epigenetic tagging of dorsal horn neurons and nocifensive behaviour
- PMID: 27482631
- PMCID: PMC5065054
- DOI: 10.1097/j.pain.0000000000000679
The mitogen and stress-activated protein kinase 1 regulates the rapid epigenetic tagging of dorsal horn neurons and nocifensive behaviour
Abstract
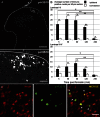
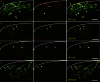
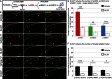
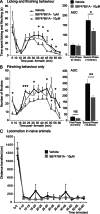
Phosphorylation of histone H3 at serine 10 (p-H3S10) is a marker of active gene transcription. Using cognitive models of neural plasticity, p-H3S10 was shown to be downstream of extracellular signal-regulated kinase (ERK) signalling in the hippocampus. In this study, we show that nociceptive signalling after peripheral formalin injection increased p-H3S10 expression in the ipsilateral dorsal horn. This increase was maximal 30 minutes after formalin injection and occurred mainly within p-ERK-positive neurons. Spinal p-H3S10-enhanced expression was also observed in neurokinin 1 receptor (NK1R), c-Fos, and Zif268 positive neurons and was inhibited by ablation of serotonergic descending controls. The mitogen and stress-activated protein kinase 1 (MSK1) is downstream of ERK and can induce p-H3S10. We found that, after formalin injection, most phospho-MSK1 (p-MSK1)-positive cells (87% ± 3%) expressed p-ERK and the majority of p-H3S10-positive cells (85% ± 5%) expressed p-MSK1. Inhibition of ERK activity with the MEK inhibitor SL327 reduced formalin-induced p-ERK, p-MSK1, and p-H3S10, demonstrating that spinal p-MSK1 and p-H3S10 were at least partly downstream of ERK signalling. Crucially, pharmacological blockade of spinal MSK1 activity with the novel MSK1 inhibitor SB727651A inhibited formalin-induced spinal p-H3S10 and nocifensive behaviour. These findings are the first to establish the involvement of p-H3S10 and its main kinase, MSK1, in ERK regulation of nociception. Given the general importance of ERK signalling in pain processing, our results suggest that p-H3S10 could play a role in the response to injury.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures


References
-
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1998;1:602–9. - PubMed
-
- Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. In: Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, Wray S, editors. Current protocols in neuroscience. Hoboken: John Wiley & Sons, Inc, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

