Peripherally Selective Cannabinoid 1 Receptor (CB1R) Agonists for the Treatment of Neuropathic Pain
- PMID: 27482723
- PMCID: PMC5474949
- DOI: 10.1021/acs.jmedchem.6b00516
Peripherally Selective Cannabinoid 1 Receptor (CB1R) Agonists for the Treatment of Neuropathic Pain
Abstract
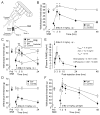
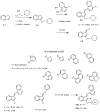
Alleviation of neuropathic pain by cannabinoids is limited by their central nervous system (CNS) side effects. Indole and indene compounds were engineered for high hCB1R affinity, peripheral selectivity, metabolic stability, and in vivo efficacy. An epithelial cell line assay identified candidates with <1% blood-brain barrier penetration for testing in a rat neuropathy induced by unilateral sciatic nerve entrapment (SNE). The SNE-induced mechanical allodynia was reversibly suppressed, partially or completely, after intraperitoneal or oral administration of several indenes. At doses that relieve neuropathy symptoms, the indenes completely lacked, while the brain-permeant CB1R agonist HU-210 (1) exhibited strong CNS side effects, in catalepsy, hypothermia, and motor incoordination assays. Pharmacokinetic findings of ∼0.001 cerebrospinal fluid:plasma ratio further supported limited CNS penetration. Pretreatment with selective CB1R or CB2R blockers suggested mainly CB1R contribution to an indene's antiallodynic effects. Therefore, this class of CB1R agonists holds promise as a viable treatment for neuropathic pain.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Ossipov MH, Porreca F. Challenges in the development of novel treatment strategies for neuropathic pain. NeuroRx. 2005;2:650–661. - PMC - PubMed
- Kinloch RA, Cox PJ. New targets for neuropathic pain therapeutics. Expert Opin Ther Targets. 2005;9:685–698. - PubMed
- Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154(Suppl1):S94–S100. - PMC - PubMed
-
- Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA. 2003;290:1757–1762. - PubMed
- Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112:299–306. - PubMed
- Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, Sansom C. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 ‘N of 1’ studies. Anaesthesia. 2004;59:440–452. - PubMed
-
- Herzberg U, Eliav E, Bennett GJ, Kopin IJ. The analgesic effects of R(+)-WIN 55;212–2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett. 1997;221:157–160. - PubMed
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. - PubMed
-
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;168:299–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

