Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury
- PMID: 27482889
- PMCID: PMC5004940
- DOI: 10.1172/JCI85939
Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury
Abstract
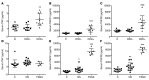
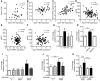
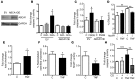
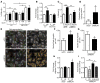
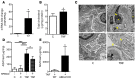
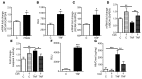
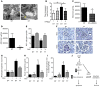
High levels of circulating TNF and its receptors, TNFR1 and TNFR2, predict the progression of diabetic kidney disease (DKD), but their contribution to organ damage in DKD remains largely unknown. Here, we investigated the function of local and systemic TNF in podocyte injury. We cultured human podocytes with sera collected from DKD patients, who displayed elevated TNF levels, and focal segmental glomerulosclerosis (FSGS) patients, whose TNF levels resembled those of healthy patients. Exogenous TNF administration or local TNF expression was equally sufficient to cause free cholesterol-dependent apoptosis in podocytes by acting through a dual mechanism that required a reduction in ATP-binding cassette transporter A1-mediated (ABCA1-mediated) cholesterol efflux and reduced cholesterol esterification by sterol-O-acyltransferase 1 (SOAT1). TNF-induced albuminuria was aggravated in mice with podocyte-specific ABCA1 deficiency and was partially prevented by cholesterol depletion with cyclodextrin. TNF-stimulated free cholesterol-dependent apoptosis in podocytes was mediated by nuclear factor of activated T cells 1 (NFATc1). ABCA1 overexpression or cholesterol depletion was sufficient to reduce albuminuria in mice with podocyte-specific NFATc1 activation. Our data implicate an NFATc1/ABCA1-dependent mechanism in which local TNF is sufficient to cause free cholesterol-dependent podocyte injury irrespective of TNF, TNFR1, or TNFR2 serum levels.
Figures

References
-
- Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72(2):165–178. doi: 10.1136/annrheumdis-2012-202545. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

