Synthetic redesign of plant lipid metabolism
- PMID: 27483205
- PMCID: PMC4982047
- DOI: 10.1111/tpj.13172
Synthetic redesign of plant lipid metabolism
Abstract
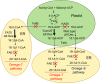
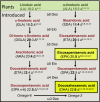
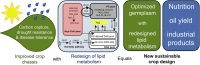
Plant seed lipid metabolism is an area of intensive research, including many examples of transgenic events in which oil composition has been modified. In the selected examples described in this review, progress towards the predictive manipulation of metabolism and the reconstitution of desired traits in a non-native host is considered. The advantages of a particular oilseed crop, Camelina sativa, as a flexible and utilitarian chassis for advanced metabolic engineering and applied synthetic biology are considered, as are the issues that still represent gaps in our ability to predictably alter plant lipid biosynthesis. Opportunities to deliver useful bio-based products via transgenic plants are described, some of which represent the most complex genetic engineering in plants to date. Future prospects are considered, with a focus on the desire to transition to more (computationally) directed manipulations of metabolism.
Keywords: fatty acid metabolism; metabolic engineering; oil crops; plant biotechnology; predictive manipulation.
© 2016 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Figures


References
-
- Bansal, S. and Durrett, T.P. (2016) Camelina sativa: an ideal platform for the metabolic engineering and field production of industrial lipids. Biochimie, 120, 9–16. - PubMed
-
- Bates, P.D. and Browse, J. (2011) The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottle neck for the accumulation of unusual fatty acids in transgenic seeds. Plant J. 68, 387–399. - PubMed
-
- Bates, P.D. , Stymne, S. and Ohlrogge, J. (2013) Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 16, 358–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

