Computational Approaches to Toll-Like Receptor 4 Modulation
- PMID: 27483231
- PMCID: PMC6274477
- DOI: 10.3390/molecules21080994
Computational Approaches to Toll-Like Receptor 4 Modulation
Abstract
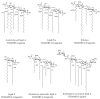
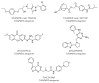
Toll-like receptor 4 (TLR4), along with its accessory protein myeloid differentiation factor 2 (MD-2), builds a heterodimeric complex that specifically recognizes lipopolysaccharides (LPS), which are present on the cell wall of Gram-negative bacteria, activating the innate immune response. Some TLR4 modulators are undergoing preclinical and clinical evaluation for the treatment of sepsis, inflammatory diseases, cancer and rheumatoid arthritis. Since the relatively recent elucidation of the X-ray crystallographic structure of the extracellular domain of TLR4, research around this fascinating receptor has risen to a new level, and thus, new perspectives have been opened. In particular, diverse computational techniques have been applied to decipher some of the basis at the atomic level regarding the mechanism of functioning and the ligand recognition processes involving the TLR4/MD-2 system at the atomic level. This review summarizes the reported molecular modeling and computational studies that have recently provided insights into the mechanism regulating the activation/inactivation of the TLR4/MD-2 system receptor and the key interactions modulating the molecular recognition process by agonist and antagonist ligands. These studies have contributed to the design and the discovery of novel small molecules with promising activity as TLR4 modulators.
Keywords: MD simulations; TLR4/MD-2 modulators; Toll-like receptor 4; computational chemistry; docking; drug design; homology modeling; molecular recognition; virtual screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







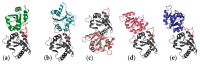
References
-
- Beutler B. TLR4 as the mammalian endotoxin sensor. Curr. Top. Microbiol. Immunol. 2002;270:109–120. - PubMed

