Measles Virus Host Invasion and Pathogenesis
- PMID: 27483301
- PMCID: PMC4997572
- DOI: 10.3390/v8080210
Measles Virus Host Invasion and Pathogenesis
Abstract
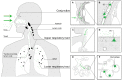
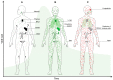
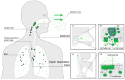
Measles virus is a highly contagious negative strand RNA virus that is transmitted via the respiratory route and causes systemic disease in previously unexposed humans and non-human primates. Measles is characterised by fever and skin rash and usually associated with cough, coryza and conjunctivitis. A hallmark of measles is the transient immune suppression, leading to increased susceptibility to opportunistic infections. At the same time, the disease is paradoxically associated with induction of a robust virus-specific immune response, resulting in lifelong immunity to measles. Identification of CD150 and nectin-4 as cellular receptors for measles virus has led to new perspectives on tropism and pathogenesis. In vivo studies in non-human primates have shown that the virus initially infects CD150⁺ lymphocytes and dendritic cells, both in circulation and in lymphoid tissues, followed by virus transmission to nectin-4 expressing epithelial cells. The abilities of the virus to cause systemic infection, to transmit to numerous new hosts via droplets or aerosols and to suppress the host immune response for several months or even years after infection make measles a remarkable disease. This review briefly highlights current topics in studies of measles virus host invasion and pathogenesis.
Keywords: immune suppression; measles virus; pathogenesis; transmission; tropism.
Figures

References
-
- Griffin D.E. Fields Virology. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. Measles virus.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

