A rat model for hepatitis E virus
- PMID: 27483350
- PMCID: PMC5087834
- DOI: 10.1242/dmm.024406
A rat model for hepatitis E virus
Abstract
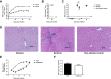
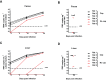
Hepatitis E virus (HEV) is one of the prime causes of acute viral hepatitis, and chronic hepatitis E is increasingly recognized as an important problem in the transplant setting. Nevertheless, the fundamental understanding of the biology of HEV replication is limited and there are few therapeutic options. The development of such therapies is partially hindered by the lack of a robust and convenient animal model. We propose the infection of athymic nude rats with the rat HEV strain LA-B350 as such a model. A cDNA clone, pLA-B350, was constructed and the infectivity of its capped RNA transcripts was confirmed in vitro and in vivo Furthermore, a subgenomic replicon, pLA-B350/luc, was constructed and validated for in vitro antiviral studies. Interestingly, rat HEV proved to be less sensitive to the antiviral activity of α-interferon, ribavirin and mycophenolic acid than genotype 3 HEV (a strain that infects humans). As a proof-of-concept, part of the C-terminal polymerase sequence of pLA-B350/luc was swapped with its genotype 3 HEV counterpart: the resulting chimeric replicon replicated with comparable efficiency as the wild-type construct, confirming that LA-B350 strain is amenable to humanization (replacement of certain sequences or motifs by their counterparts from human HEV strains). Finally, ribavirin effectively inhibited LA-B350 replication in athymic nude rats, confirming the suitability of the rat model for antiviral studies.
Keywords: Animal model; Antiviral; Hepatitis E virus; LA-B350; Rat; Ribavirin.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
-
- Debing Y., Emerson S. U., Wang Y., Pan Q., Balzarini J., Dallmeier K. and Neyts J. (2014b). Ribavirin inhibits in vitro hepatitis E virus replication through depletion of cellular GTP pools and is moderately synergistic with alpha interferon. Antimicrob. Agents Chemother. 58, 267-273. 10.1128/AAC.01795-13 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

