Alterations in nuclear structure promote lupus autoimmunity in a mouse model
- PMID: 27483354
- PMCID: PMC5007980
- DOI: 10.1242/dmm.024851
Alterations in nuclear structure promote lupus autoimmunity in a mouse model
Abstract
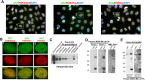
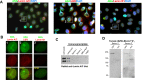
Systemic lupus erythematosus (SLE) is an autoimmune disorder characterized by the development of autoantibodies that recognize components of the cell nucleus. The vast majority of lupus research has focused on either the contributions of immune cell dysfunction or the genetics of the disease. Because granulocytes isolated from human SLE patients had alterations in neutrophil nuclear morphology that resembled the Pelger-Huet anomaly, and had prominent mis-splicing of mRNA encoding the nuclear membrane protein lamin B receptor (LBR), consistent with their Pelger-Huet-like nuclear morphology, we used a novel mouse model system to test the hypothesis that a disruption in the structure of the nucleus itself also contributes to the development of lupus autoimmunity. The lupus-prone mouse strain New Zealand White (NZW) was crossed with c57Bl/6 mice harboring a heterozygous autosomal dominant mutation in Lbr (B6.Lbr(ic/+)), and the (NZW×B6.Lbr(ic))F1 offspring were evaluated for induction of lupus autoimmunity. Only female (NZW×B6.Lbr(ic))F1 mice developed lupus autoimmunity, which included splenomegaly, kidney damage and autoantibodies. Kidney damage was accompanied by immune complex deposition, and perivascular and tubule infiltration of mononuclear cells. The titers of anti-chromatin antibodies exceeded those of aged female MRL-Fas(lpr) mice, and were predominantly of the IgG2 subclasses. The anti-nuclear antibody staining profile of female (NZW×B6.Lbr(ic))F1 sera was complex, and consisted of an anti-nuclear membrane reactivity that colocalized with the A-type lamina, in combination with a homogeneous pattern that was related to the recognition of histones with covalent modifications that are associated with gene activation. An anti-neutrophil IgM recognizing calreticulin, but not myeloperoxidase (MPO) or proteinase 3 (PR3), was also identified. Thus, alterations in nuclear structure contribute to lupus autoimmunity when expressed in the context of a lupus-prone genetic background, suggesting a mechanism for the development of lupus autoimmunity in genetically predisposed individuals that is induced by the disruption of nuclear architecture.
Keywords: Autoantibody; Calreticulin; Chromatin; Histone modifications; Lamina; Nucleus.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures





References
-
- Armstrong D. L., Zidovetzki R., Alarcón-Riquelme M. E., Tsao B. P., Criswell L. A., Kimberly R. P., Harley J. B., Sivils K. L., Vyse T. J., Gaffney P. M. et al. (2014). GWAS identifies novel SLE susceptibility genes and explains the association of the HLA region. Genes Immun. 15, 347-354. 10.1038/gene.2014.23 - DOI - PMC - PubMed
-
- Binda O., LeRoy G., Bua D. J., Garcia B. A., Gozani O. and Richard S. (2010). Trimethylation of histone H3 lysine 4 impairs methylation of histone H3 lysine 9: regulation of lysine methyltransferases by physical interaction with their substrates. Epigenetics 5, 767-775. 10.4161/epi.5.8.13278 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

