Adverse Renal, Endocrine, Hepatic, and Metabolic Events during Maintenance Mood Stabilizer Treatment for Bipolar Disorder: A Population-Based Cohort Study
- PMID: 27483368
- PMCID: PMC4970809
- DOI: 10.1371/journal.pmed.1002058
Adverse Renal, Endocrine, Hepatic, and Metabolic Events during Maintenance Mood Stabilizer Treatment for Bipolar Disorder: A Population-Based Cohort Study
Abstract
Background: There is limited, poorly characterized information about adverse events occurring during maintenance treatment of bipolar disorder. We aimed to determine adverse event rates during treatment with lithium, valproate, olanzapine, and quetiapine.
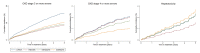
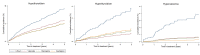
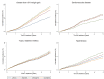
Methods and findings: We conducted a propensity score adjusted cohort study using nationally representative United Kingdom electronic health records from January 1, 1995, until December 31, 2013. We included patients who had a diagnosis of bipolar disorder and were prescribed lithium (n = 2148), valproate (n = 1670), olanzapine (n = 1477), or quetiapine (n = 1376) as maintenance mood stabilizer treatment. Adverse outcomes were chronic kidney disease, thyroid disease, hypercalcemia, weight gain, hypertension, type 2 diabetes mellitus, cardiovascular disease, and hepatotoxicity. The propensity score included important demographic, physical health, and mental health predictors of drug treatment allocation. The median duration of drug treatment was 1.48 y (interquartile range 0.64-3.43). Compared to patients prescribed lithium, those taking valproate, olanzapine, and quetiapine had reduced rates of chronic kidney disease stage 3 or more severe, following adjustment for propensity score, age, and calendar year, and accounting for clustering by primary care practice (valproate hazard ratio [HR] 0.56; 95% confidence interval [CI] 0.45-0.69; p < 0.001, olanzapine HR 0.57; 95% CI 0.45-0.71; p < 0.001, quetiapine HR 0.62; 95% CI 0.47-0.80; p < 0.001). Hypothyroidism was reduced in those taking valproate (HR 0.60; 95% CI 0.40-0.89; p = 0.012) and olanzapine (HR 0.48; 95% CI 0.29-0.77; p = 0.003), compared to those taking lithium. Rates of new onset hyperthyroidism (valproate HR 0.24; 95% CI 0.09-0.61; p = 0.003, olanzapine HR 0.31; 95% CI 0.13-0.73; p = 0.007) and hypercalcemia (valproate HR 0.25; 95% CI 0.10-0.60; p = 0.002, olanzapine HR 0.32; 95% CI 0.14-0.76; p = 0.008, quetiapine HR 0.23; 95% CI 0.07-0.73; p = 0.013) were also reduced relative to lithium. However, rates of greater than 15% weight gain on valproate, olanzapine, and quetiapine were higher (valproate HR 1.62; 95% CI 1.31-2.01; p < 0.001, olanzapine HR 1.84; 95% CI 1.47-2.30; p < 0.001, quetiapine HR 1.67; 95% CI 1.24-2.20; p < 0.001) than in individuals prescribed lithium, as were rates of hypertension in the olanzapine treated group (HR 1.41, 95% CI 1.06-1.87; p = 0.017). We found no significant difference in rates of chronic kidney disease stage 4 or more severe, type 2 diabetes mellitus, cardiovascular disease, or hepatotoxicity. Despite estimates being robust following sensitivity analyses, limitations include the potential for residual confounding and ascertainment bias and an inability to examine dosage effects.
Conclusions: Lithium use is associated with more renal and endocrine adverse events but less weight gain than commonly used alternative mood stabilizers. Risks need to be offset with the effectiveness and anti-suicidal benefits of lithium and the potential metabolic side effects of alternative treatment options.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Comment in
-
Adverse events associated with mood stabiliser treatment should be continuously monitored in patients diagnosed with bipolar affective disorder.Evid Based Med. 2017 Apr;22(2):74-75. doi: 10.1136/ebmed-2016-110629. Epub 2017 Mar 2. Evid Based Med. 2017. PMID: 28254753 No abstract available.
References
-
- National Institute for Health and Care Excellence. Bipolar Disorder: the Management of Bipolar Disorder in Adults, Children and Adolescents, in Primary and Secondary Care. GC185. London, UK: NICE; 2014.
-
- Miura T, Noma H, Furukawa TA, Mitsuyasu H, Tanaka S, Stockton S, et al. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2014;1(5):351–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

