Acceptability and feasibility of point-of-care CD4 testing on HIV continuum of care in low and middle income countries: a systematic review
- PMID: 27484023
- PMCID: PMC4971709
- DOI: 10.1186/s12913-016-1588-y
Acceptability and feasibility of point-of-care CD4 testing on HIV continuum of care in low and middle income countries: a systematic review
Abstract
Background: CD4 testing is, and will remain an important part of HIV treatment and care in low and middle income countries (LMICs). We report the findings of a systematic review assessing acceptability and feasibility of POC CD4 testing in field settings.
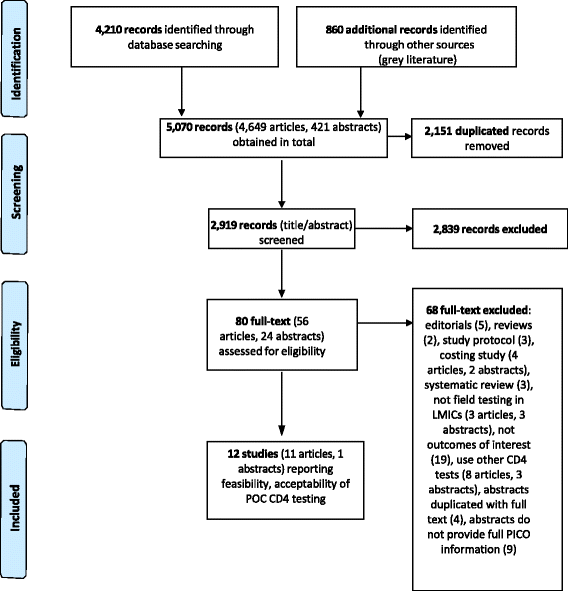
Methods: Electronic databases were searched for studies published in English between 2005 and 2015 that describe POC CD4 platforms. Studies conducted in LMICs and under field conditions outside a laboratory environment were eligible. Qualitative and descriptive data analysis was used to present the findings.
Results: Twelve studies were included, 11 of which were conducted in sub-Saharan countries and used one POC CD4 test (The Alere Pima CD4). Patients reported positively regarding the implementation of POC CD4 testing at primary health care and community level with ≥90 % of patients accepting the test across various study settings. Health service providers expressed preference toward POC CD4 testing as it is easy-to-use, efficient and satisfied patients' needs to a greater extent as compared to conventional methods. However, operational challenges including preference toward venous blood rather than finger-prick sampling, frequent device failures and operator errors, quality of training for test operators and supervisors, and increased staff workload were also identified.
Conclusions: POC CD4 testing seems acceptable and feasible in LIMCs under field conditions. Further studies using different POC CD4 tests available on the market are required to provide critical data to support countries in selection and implementation of appropriate POC CD4 technologies.
Keywords: CD4; Pima; Point-of-care testing; acceptability; feasibility; systematic review.
Figures
Similar articles
-
Performance of point-of-care CD4 testing technologies in resource-constrained settings: a systematic review and meta-analysis.BMC Infect Dis. 2016 Oct 21;16(1):592. doi: 10.1186/s12879-016-1931-2. BMC Infect Dis. 2016. PMID: 27769181 Free PMC article.
-
Point-of-care CD4+ technology implementation in Free State, South Africa, was associated with improved patient health outcomes.S Afr Med J. 2020 Jan 29;110(2):126-131. doi: 10.7196/SAMJ.2020.v110i2.13823. S Afr Med J. 2020. PMID: 32657683
-
Performance of the BD FACSPresto near to patient analyzer in comparison with representative conventional CD4 instruments in Cameroon.AIDS Res Ther. 2020 Aug 17;17(1):53. doi: 10.1186/s12981-020-00309-9. AIDS Res Ther. 2020. PMID: 32799909 Free PMC article.
-
Laboratory-based performance evaluation of PIMA CD4+ T-lymphocyte count point-of-care by lay-counselors in Kenya.J Immunol Methods. 2017 Sep;448:44-50. doi: 10.1016/j.jim.2017.05.006. Epub 2017 May 18. J Immunol Methods. 2017. PMID: 28529048 Free PMC article.
-
Thirty-five years of CD4 T-cell counting in HIV infection: From flow cytometry in the lab to point-of-care testing in the field.Cytometry B Clin Cytom. 2017 Nov;92(6):437-444. doi: 10.1002/cyto.b.21400. Epub 2016 Aug 3. Cytometry B Clin Cytom. 2017. PMID: 27406947 Review.
Cited by
-
Left behind on the path to 90-90-90: understanding and responding to HIV among displaced people.J Int AIDS Soc. 2022 Nov;25(11):e26031. doi: 10.1002/jia2.26031. J Int AIDS Soc. 2022. PMID: 36352546 Free PMC article.
-
Feasibility and acceptability of implementing early infant diagnosis of HIV in Papua New Guinea at the point of care: a qualitative exploration of health worker and key informant perspectives.BMJ Open. 2020 Nov 19;10(11):e043679. doi: 10.1136/bmjopen-2020-043679. BMJ Open. 2020. PMID: 33444219 Free PMC article. Clinical Trial.
-
Forecasting the global demand for HIV monitoring and diagnostic tests: A 2016-2021 analysis.PLoS One. 2018 Sep 19;13(9):e0201341. doi: 10.1371/journal.pone.0201341. eCollection 2018. PLoS One. 2018. PMID: 30231022 Free PMC article.
-
Assessing Very Early Infant Diagnosis Turnaround Times: Findings from a Birth Testing Pilot in Lesotho.AIDS Res Treat. 2017;2017:2572594. doi: 10.1155/2017/2572594. Epub 2017 Dec 19. AIDS Res Treat. 2017. PMID: 29410914 Free PMC article.
-
A Prospective Evaluation of the Diagnostic Accuracy of the Point-of-Care VISITECT CD4 Advanced Disease Test in 7 Countries.J Infect Dis. 2025 Feb 4;231(1):e82-e90. doi: 10.1093/infdis/jiae374. J Infect Dis. 2025. PMID: 39046150 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials