Origins of tmRNA: the missing link in the birth of protein synthesis?
- PMID: 27484476
- PMCID: PMC5041485
- DOI: 10.1093/nar/gkw693
Origins of tmRNA: the missing link in the birth of protein synthesis?
Abstract
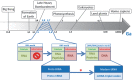
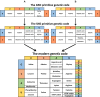
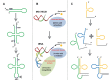
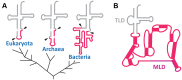
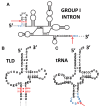
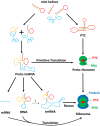
The RNA world hypothesis refers to the early period on earth in which RNA was central in assuring both genetic continuity and catalysis. The end of this era coincided with the development of the genetic code and protein synthesis, symbolized by the apparition of the first non-random messenger RNA (mRNA). Modern transfer-messenger RNA (tmRNA) is a unique hybrid molecule which has the properties of both mRNA and transfer RNA (tRNA). It acts as a key molecule during trans-translation, a major quality control pathway of modern bacterial protein synthesis. tmRNA shares many common characteristics with ancestral RNA. Here, we present a model in which proto-tmRNAs were the first molecules on earth to support non-random protein synthesis, explaining the emergence of early genetic code. In this way, proto-tmRNA could be the missing link between the first mRNA and tRNA molecules and modern ribosome-mediated protein synthesis.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Crick F.H. The origin of the genetic code. J. Mol. Biol. 1968;38:367–379. - PubMed
-
- Orgel L.E. Evolution of the genetic apparatus. J. Mol. Biol. 1968;38:381–393. - PubMed
-
- Woese C.R. Harper & Row. 1967. The genetic code the molecular basis for genetic expression. ASIN: B000I3M2KK.
-
- Jeffares D.C., Poole A.M., Penny D. Relics from the RNA world. J. Mol. Evol. 1998;46:18–36. - PubMed
-
- Goldberg A.L., Wittes R.E. Genetic code: aspects of organization. Science. 1966;153:420–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

