Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: the HALT-PKD clinical trial
- PMID: 27484667
- PMCID: PMC5837227
- DOI: 10.1093/ndt/gfw294
Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: the HALT-PKD clinical trial
Abstract
Background: Patients with mild autosomal dominant polycystic kidney disease (ADPKD) are less likely to be informative in randomized clinical trials (RCTs). We previously developed an imaging classification of ADPKD (typical diffuse cyst distribution Class 1A-E and atypical cyst distribution Class 2) for prognostic enrichment design in RCTs. We investigated whether using this classification would have increased the power to detect a beneficial treatment effect of rigorous blood pressure (BP) control on HALT-PKD participants with early disease (Study A).
Methods: Post hoc analysis of the early disease HALT-PKD study, an RCT that studied the effect of rigorous versus standard BP control on rates of total kidney volume (TKV) increase and estimated glomerular filtration rate (eGFR) decline in ADPKD patients with eGFR >60 mL/min/1.73 m2.
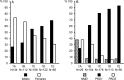
Results: Five hundred and fifty-one patients were classified by two observers (98.2% agreement) into Class 1A (6.2%), 1B (20.3%), 1C (34.1%), 1D (22.1%), 1E (11.8%) and 2 (5.4%). The TKV increase and eGFR decline became steeper from Class 1A through 1E. Rigorous BP control had been shown to be associated with slower TKV increase, without a significant overall effect on the rate of eGFR decline (faster in the first 4 months and marginally slower thereafter). Merging Classes 1A and 2 (lowest severity), 1B and 1C (intermediate severity) and 1D and 1E (highest severity) detected stronger beneficial effects on TKV increase and eGFR decline in Class 1D and E with a smaller number of patients.
Conclusions: Strategies for prognostic enrichment, such as image classification, should be used in the design of RCTs for ADPKD to increase their power and reduce their cost.
Keywords: HALT-PKD study; autosomal dominant polycystic kidney disease; eGFR; image classification; total kidney volume.
© The Author 2016. Published by Oxford University Press on behalf of ERA-EDTA. All rights reserved.
Figures


References
-
- Ruggenenti P, Remuzzi A, Ondei P. et al. Safety and efficacy of long-acting somatostatin treatment in autosomal dominant polcysytic kidney disease. Kidney Int 2005; 68: 206–216 - PubMed
-
- van Keimpema L, Nevens F, Vanslembrouck R. et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2009; 137: 1661–1668e1–2 - PubMed
MeSH terms
Grants and funding
- U01 DK062401/DK/NIDDK NIH HHS/United States
- P30 DK090728/DK/NIDDK NIH HHS/United States
- U01 DK062402/DK/NIDDK NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- P30 DK106912/DK/NIDDK NIH HHS/United States
- UL1 TR000001/TR/NCATS NIH HHS/United States
- M01 RR001032/RR/NCRR NIH HHS/United States
- UL1 TR000135/TR/NCATS NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- U01 DK062411/DK/NIDDK NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- U01 DK062410/DK/NIDDK NIH HHS/United States
- M01 RR000585/RR/NCRR NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- M01 RR000039/RR/NCRR NIH HHS/United States
- UL1 RR033179/RR/NCRR NIH HHS/United States
- M01 RR000054/RR/NCRR NIH HHS/United States
- M01 RR023940/RR/NCRR NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- U01 DK062408/DK/NIDDK NIH HHS/United States
- R01 DK044863/DK/NIDDK NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- U01 DK082230/DK/NIDDK NIH HHS/United States
- UL1 RR024150/RR/NCRR NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

