Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser
- PMID: 27484673
- PMCID: PMC4971496
- DOI: 10.1038/srep30540
Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser
Abstract
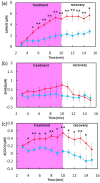
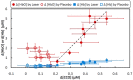
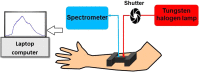
Photobiomodulation, also known as low-level laser/light therapy (LLLT), refers to the use of red-to-near-infrared light to stimulate cellular functions for physiological or clinical benefits. The mechanism of LLLT is assumed to rely on photon absorption by cytochrome c oxidase (CCO), the terminal enzyme in the mitochondrial respiratory chain that catalyzes the reduction of oxygen for energy metabolism. In this study, we used broadband near-infrared spectroscopy (NIRS) to measure the LLLT-induced changes in CCO and hemoglobin concentrations in human forearms in vivo. Eleven healthy participants were administered with 1064-nm laser and placebo treatments on their right forearms. The spectroscopic data were analyzed and fitted with wavelength-dependent, modified Beer-Lambert Law. We found that LLLT induced significant increases of CCO concentration (Δ[CCO]) and oxygenated hemoglobin concentration (Δ[HbO]) on the treated site as the laser energy dose accumulated over time. A strong linear interplay between Δ[CCO] and Δ[HbO] was observed for the first time during LLLT, indicating a hemodynamic response of oxygen supply and blood volume closely coupled to the up-regulation of CCO induced by photobiomodulation. These results demonstrate the tremendous potential of broadband NIRS as a non-invasive, in vivo means to study mechanisms of photobiomodulation and perform treatment evaluations of LLLT.
Figures


References
-
- Chow R. T., Johnson M. I., Lopes-Martins R. A. & Bjordal J. M. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 374, 1897–1908, 10.1016/S0140-6736(09)61522-1 (2009). - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

