Ligand-induced Epitope Masking: DISSOCIATION OF INTEGRIN α5β1-FIBRONECTIN COMPLEXES ONLY BY MONOCLONAL ANTIBODIES WITH AN ALLOSTERIC MODE OF ACTION
- PMID: 27484800
- PMCID: PMC5076510
- DOI: 10.1074/jbc.M116.736942
Ligand-induced Epitope Masking: DISSOCIATION OF INTEGRIN α5β1-FIBRONECTIN COMPLEXES ONLY BY MONOCLONAL ANTIBODIES WITH AN ALLOSTERIC MODE OF ACTION
Abstract
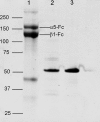
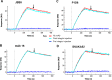
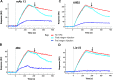
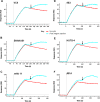
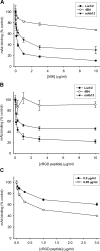
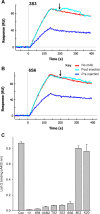
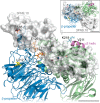
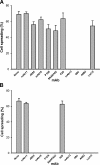
We previously demonstrated that Arg-Gly-Asp (RGD)-containing ligand-mimetic inhibitors of integrins are unable to dissociate pre-formed integrin-fibronectin complexes (IFCs). These observations suggested that amino acid residues involved in integrin-fibronectin binding become obscured in the ligand-occupied state. Because the epitopes of some function-blocking anti-integrin monoclonal antibodies (mAbs) lie near the ligand-binding pocket, it follows that the epitopes of these mAbs may become shielded in the ligand-occupied state. Here, we tested whether function-blocking mAbs directed against α5β1 can interact with the integrin after it forms a complex with an RGD-containing fragment of fibronectin. We showed that the anti-α5 subunit mAbs JBS5, SNAKA52, 16, and P1D6 failed to disrupt IFCs and hence appeared unable to bind to the ligand-occupied state. In contrast, the allosteric anti-β1 subunit mAbs 13, 4B4, and AIIB2 could dissociate IFCs and therefore were able to interact with the ligand-bound state. However, another class of function-blocking anti-β1 mAbs, exemplified by Lia1/2, could not disrupt IFCs. This second class of mAbs was also distinguished from 13, 4B4, and AIIB2 by their ability to induce homotypic cell aggregation. Although the epitope of Lia1/2 was closely overlapping with those of 13, 4B4, and AIIB2, it appeared to lie closer to the ligand-binding pocket. A new model of the α5β1-fibronectin complex supports our hypothesis that the epitopes of mAbs that fail to bind to the ligand-occupied state lie within, or very close to, the integrin-fibronectin interface. Importantly, our findings imply that the efficacy of some therapeutic anti-integrin mAbs could be limited by epitope masking.
Keywords: allosteric regulation; antibody; cell adhesion; epitope masking; fibronectin; integrin; therapeutics.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Hynes R. O. (2002) Integrins: bidirectional, allosteric signalling machines. Cell 110, 673–687 - PubMed
-
- Goodman S. L., and Picard M. (2012) Integrins as therapeutic targets. Trends Pharmacol. Sci. 33, 405–412 - PubMed
-
- Giordano A., Musumeci G., D'Angelillo A., Rossini R., Zoccai G. B., Messina S., Coscioni E., Romano S., and Romano M. F. (2016) Effects of glycoprotein IIb/IIIa antagonists: anti platelet aggregation and beyond. Curr. Drug Metab. 17, 194–203 - PubMed
-
- Rice G. P., Hartung H. P., and Calabresi P. A. (2005) Anti-α4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology 64, 1336–1342 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

