Optogenetic and chemogenetic strategies for sustained inhibition of pain
- PMID: 27484850
- PMCID: PMC4971509
- DOI: 10.1038/srep30570
Optogenetic and chemogenetic strategies for sustained inhibition of pain
Abstract
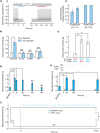
Spatially targeted, genetically-specific strategies for sustained inhibition of nociceptors may help transform pain science and clinical management. Previous optogenetic strategies to inhibit pain have required constant illumination, and chemogenetic approaches in the periphery have not been shown to inhibit pain. Here, we show that the step-function inhibitory channelrhodopsin, SwiChR, can be used to persistently inhibit pain for long periods of time through infrequent transdermally delivered light pulses, reducing required light exposure by >98% and resolving a long-standing limitation in optogenetic inhibition. We demonstrate that the viral expression of the hM4D receptor in small-diameter primary afferent nociceptor enables chemogenetic inhibition of mechanical and thermal nociception thresholds. Finally, we develop optoPAIN, an optogenetic platform to non-invasively assess changes in pain sensitivity, and use this technique to examine pharmacological and chemogenetic inhibition of pain.
Conflict of interest statement
S.M.I. and S.L.D. have filed a patent application related to methods described in this paper.
Figures


References
-
- Nelson L. S., Juurlink D. N. & Perrone J. Addressing the Opioid Epidemic. JAMA 314, 1453–1454 (2015). - PubMed
-
- Han B., Compton W. M., Jones C. M. & Cai R. Nonmedical Prescription Opioid Use and Use Disorders Among Adults Aged 18 Through 64 Years in the United States, 2003–2013. JAMA 314, 1468–1478 (2015). - PubMed
-
- Snowball A. & Schorge S. Changing channels in pain and epilepsy: Exploiting ion channel gene therapy for disorders of neuronal hyperexcitability. FEBS Lett. 589, 1620–1634 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

