KIF1A mediates axonal transport of BACE1 and identification of independently moving cargoes in living SCG neurons
- PMID: 27484852
- PMCID: PMC5132087
- DOI: 10.1111/tra.12428
KIF1A mediates axonal transport of BACE1 and identification of independently moving cargoes in living SCG neurons
Abstract
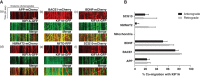
Neurons rely heavily on axonal transport to deliver materials from the sites of synthesis to the axon terminals over distances that can be many centimetres long. KIF1A is the neuron-specific kinesin with the fastest reported anterograde motor activity. Previous studies have shown that KIF1A transports a subset of synaptic proteins, neurofilaments and dense-core vesicles. Using two-colour live imaging, we showed that beta-secretase 1 (BACE1)-mCherry moves together with KIF1A-GFP in both the anterograde and retrograde directions in superior cervical ganglions (SCG) neurons. We confirmed that KIF1A is functionally required for BACE1 transport by using KIF1A siRNA and a KIF1A mutant construct (KIF1A-T312M) to impair its motor activity. We further identified several cargoes that have little or no co-migration with KIF1A-GFP and also move independently from BACE1-mCherry. Together, these findings support a primary role for KIF1A in the anterograde transport of BACE1 and suggest that axonally transported cargoes are sorted into different classes of carrier vesicles in the cell body and are transported by cargo-specific motor proteins through the axon.
Keywords: BACE1; KIF1A; axonal transport; live-cell imaging.
© 2016 The Authors. Traffic published by John Wiley & Sons Ltd.
Figures







References
-
- Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10(10):682–696. - PubMed
-
- Shah SB, Yang G, Danuser G, Goldstein LS. Axonal Transport: Imaging and Modeling of a Neuronal Process. Controlled Nanoscale Motion Springer; 2007:65–84.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

