Epigenetic and Glucocorticoid Receptor-Mediated Regulation of Glutathione Peroxidase 3 in Lung Cancer Cells
- PMID: 27484907
- PMCID: PMC4990756
- DOI: 10.14348/molcells.2016.0164
Epigenetic and Glucocorticoid Receptor-Mediated Regulation of Glutathione Peroxidase 3 in Lung Cancer Cells
Abstract
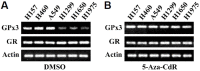
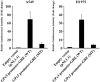
Glutathione peroxidase 3 (GPx3), an antioxidant enzyme, acts as a modulator of redox signaling, has immunomodulatory function, and catalyzes the detoxification of reactive oxygen species (ROS). GPx3 has been identified as a tumor suppressor in many cancers. Although hyper-methylation of the GPx3 promoter has been shown to down-regulate its expression, other mechanisms by which GPx3 expression is regulated have not been reported. The aim of this study was to further elucidate the mechanisms of GPx3 regulation. GPx3 gene analysis predicted the presence of ten glucocorticoid response elements (GREs) on the GPx3 gene. This result prompted us to investigate whether GPx3 expression is regulated by the glucocorticoid receptor (GR), which is implicated in tumor response to chemotherapy. The corticosteroid dexamethasone (Dex) was used to examine the possible relationship between GR and GPx3 expression. Dex significantly induced GPx3 expression in H1299, H1650, and H1975 cell lines, which exhibit low levels of GPx3 expression under normal conditions. The results of EMSA and ChIP-PCR suggest that GR binds directly to GRE 6 and 7, both of which are located near the GPx3 promoter. Assessment of GPx3 transcription efficiency using a luciferase reporter system showed that blocking formation of the GR-GRE complexes reduced luciferase activity by 7-8-fold. Suppression of GR expression by siRNA transfection also induced down-regulation of GPx3. These data indicate that GPx3 expression can be regulated independently via epigenetic or GR-mediated mechanisms in lung cancer cells, and suggest that GPx3 could potentiate glucocorticoid (GC)-mediated anti-inflammatory signaling in lung cancer cells.
Keywords: dexamethasone; gene regulation; glucocorticoid receptor; glucocorticoid response element; glucocorticoids; glutathione peroxidase-3.
Figures




References
-
- An B.C., Lee S.S., Jung H.S., Kim J.Y., Lee Y., Lee K.W., Lee S.Y., Tripathi B.N., Chung B.Y. An additional cysteine in a typical 2-Cys peroxiredoxin of Pseudomonas promotes functional switching between peroxidase and molecular chaperone. FEBS Lett. 2015;589:2831–2840. - PubMed
-
- Bibikova M., Barnes B., Tsan C., Ho V., Klotzle B., Le J.M., Delano D., Zhang L., Schroth G.P., Gunderson K.L., et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98:288–295. - PubMed
-
- Bierl C., Voetsch B., Jin R.C., Handy D.E., Loscalzo J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J. Biol. Chem. 2004;279:26839–26845. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

