Loss of coxsackie and adenovirus receptor expression in human colorectal cancer: A potential impact on the efficacy of adenovirus-mediated gene therapy in Chinese Han population
- PMID: 27485384
- PMCID: PMC4991754
- DOI: 10.3892/mmr.2016.5536
Loss of coxsackie and adenovirus receptor expression in human colorectal cancer: A potential impact on the efficacy of adenovirus-mediated gene therapy in Chinese Han population
Abstract
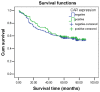
The coxsackie and adenovirus receptor (CAR) is considered a tumor suppressor and critical factor for the efficacy of therapeutic strategies that employ the adenovirus. However, data on CAR expression levels in colorectal cancer are conflicting and its clinical relevance remains to be elucidated. Immunohistochemistry was performed on tissue microarrays containing 251 pairs of colon cancer and adjacent normal tissue samples from Chinese Han patients to assess the expression levels of CAR. Compared with healthy mucosa, decreased CAR expression (40.6% vs. 95.6%; P<0.001) was observed in colorectal cancer samples. The CAR immunopositivity in tumor tissues was not significantly associated with gender, age, tumor size, differentiation, TNM stage, lymph node metastasis or distant metastasis in patients with colon cancer. However, expression of CAR is present in 83.3% of the tumor tissues from patient with colorectal liver metastasis, which was significantly higher than those without liver metastasis (39.6%; P=0.042). At the plasma membrane, CAR was observed in 29.5% normal mucosa samples, which was significantly higher than in colorectal cancer samples (4.0%; P<0.001). In addition, the survival analysis demonstrated that the expression level of CAR has no association with the prognosis of colorectal cancer. CAR expression was observed to be downregulated in colorectal cancer, and it exerts complex effects during colorectal carcinogenesis, potentially depending on the stage of the cancer development and progression. High CAR expression may promote liver metastasis. With regard to oncolytic therapy, CAR expression analysis should be performed prior to adenoviral oncolytic treatment to stratify Chinese Han patients for treatment.
Figures

Similar articles
-
Expression of coxsackie and adenovirus receptor is correlated with inferior prognosis in liver cancer patients.Oncol Lett. 2019 Feb;17(2):2485-2490. doi: 10.3892/ol.2018.9868. Epub 2018 Dec 24. Oncol Lett. 2019. PMID: 30719117 Free PMC article.
-
Targeting CD46 Enhances Anti-Tumoral Activity of Adenovirus Type 5 for Bladder Cancer.Int J Mol Sci. 2018 Sep 10;19(9):2694. doi: 10.3390/ijms19092694. Int J Mol Sci. 2018. PMID: 30201920 Free PMC article.
-
Enhanced therapeutic efficacy of an adenovirus-PEI-bile-acid complex in tumors with low coxsackie and adenovirus receptor expression.Biomaterials. 2014 Jul;35(21):5505-16. doi: 10.1016/j.biomaterials.2014.03.060. Epub 2014 Apr 13. Biomaterials. 2014. PMID: 24731708
-
The coxsackievirus and adenovirus receptor: virological and biological beauty.FEBS Lett. 2020 Jun;594(12):1828-1837. doi: 10.1002/1873-3468.13794. Epub 2020 May 9. FEBS Lett. 2020. PMID: 32298477 Review.
-
CAR: A key regulator of adhesion and inflammation.Int J Biochem Cell Biol. 2017 Aug;89:1-5. doi: 10.1016/j.biocel.2017.05.025. Epub 2017 May 22. Int J Biochem Cell Biol. 2017. PMID: 28545889 Review.
Cited by
-
Overcoming the limitations of locally administered oncolytic virotherapy.BMC Biomed Eng. 2019 Jul 1;1:17. doi: 10.1186/s42490-019-0016-x. eCollection 2019. BMC Biomed Eng. 2019. PMID: 32903299 Free PMC article. Review.
-
Precision virotherapies: Coming soon.Oncotarget. 2018 Nov 2;9(86):35605-35606. doi: 10.18632/oncotarget.26280. eCollection 2018 Nov 2. Oncotarget. 2018. PMID: 30479689 Free PMC article. No abstract available.
-
Polymeric Systems for Cancer Immunotherapy: A Review.Front Immunol. 2022 Feb 22;13:826876. doi: 10.3389/fimmu.2022.826876. eCollection 2022. Front Immunol. 2022. PMID: 35273607 Free PMC article. Review.
-
Receptors and Host Factors for Enterovirus Infection: Implications for Cancer Therapy.Cancers (Basel). 2024 Sep 12;16(18):3139. doi: 10.3390/cancers16183139. Cancers (Basel). 2024. PMID: 39335111 Free PMC article. Review.
-
Adenovirus Receptor Expression in Cancer and Its Multifaceted Role in Oncolytic Adenovirus Therapy.Int J Mol Sci. 2020 Sep 17;21(18):6828. doi: 10.3390/ijms21186828. Int J Mol Sci. 2020. PMID: 32957644 Free PMC article. Review.
References
-
- Rayburn ER, Wang W, Zhang R, Wang H. Experimental therapy for colon cancer: Anti-cancer effects of TLR9 agonism, combination with other therapeutic modalities, and dependence upon p53. Int J Oncol. 2007;30:1511–1519. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

