Synthesis and evaluation of an (18)F-labeled pyrimidine-pyridine amine for targeting CXCR4 receptors in gliomas
- PMID: 27485481
- PMCID: PMC5363724
- DOI: 10.1016/j.nucmedbio.2016.05.005
Synthesis and evaluation of an (18)F-labeled pyrimidine-pyridine amine for targeting CXCR4 receptors in gliomas
Abstract
Introduction: Chemokine receptor-4 (CXCR4, fusin, CD184) is expressed on several tissues involved in immune regulation and is upregulated in many diseases including malignant gliomas. A radiolabeled small molecule that readily crosses the blood-brain barrier can aid in identifying CXCR4-expressing gliomas and monitoring CXCR4-targeted therapy. In the current work, we have synthesized and evaluated an [(18)F]-labeled small molecule based on a pyrimidine-pyridine amine for its ability to target CXCR4.
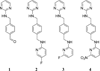
Experimental: The nonradioactive standards and the nitro precursor used in this study were prepared using established methods. An HPLC method was developed to separate the nitro-precursor from the nonradioactive standard and radioactive product. The nitro-precursor was radiolabeled with (18)F under inert, anhydrous conditions using the [(18)F]-kryptofix 2.2.2 complex to form the desired N-(4-(((6-[(18)F]fluoropyridin-2-yl)amino)methyl)benzyl)pyrimidin-2-amine ([(18)F]-3). The purified radiolabeled compound was used in serum stability, partition coefficient, cellular uptake, and in vivo cancer targeting studies.
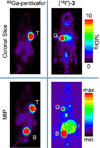
Results: [(18)F]-3 was synthesized in 4-10% decay-corrected yield (to start of synthesis). [(18)F]-3 (tR ≈ 27 min) was separated from the precursor (tR ≈ 30 min) using a pentafluorophenyl column with an isocratic solvent system. [(18)F]-3 displayed acceptable serum stability over 2 h. The amount of [(18)F]-3 bound to the plasma proteins was determined to be > 97%. The partition coefficient (LogD7.4) is 1.4 ± 0.5. Competitive in vitro inhibition indicated 3 does not inhibit uptake of (67)Ga-pentixafor. Cell culture media incubation and ex vivo urine analysis indicate rapid metabolism of [(18)F]-3 into hydrophilic metabolites. Thus, in vitro uptake of [(18)F]-3 in CXCR4 overexpressing U87 cells (U87 CXCR4) and U87 WT indicated no specific binding. In vivo studies in mice bearing U87 CXCR4 and U87 WT tumors on the left and right shoulders were carried out using [(18)F]-3 and (68)Ga-pentixafor on consecutive days. The CXCR4 positive tumor was clearly visualized in the PET study using (68)Ga-pentixafor, but not with [(18)F]-3.
Conclusions: We have successfully synthesized both a radiolabeled analog to previously reported CXCR4-targeting molecules and a nitro precursor. Our in vitro and in vivo studies indicate that [(18)F]-3 is rapidly metabolized and, therefore, does not target CXCR4-expressing tumors. Optimization of the structure to improve the in vivo (and in vitro) stability, binding, and solubility could lead to an appropriate CXCR4-targeted radiodiagnositic molecule.
Keywords: (18)F; CXCR4; Dipyrimidine amine; Pyrimidine-pyridine amine; Radiosynthesis.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
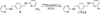


References
-
- Zou YR, Kottman AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in heaematopolesis and in cerebellar development. Nature. 1998;393:595–599. - PubMed
-
- Ogłodek EA, Szota AM, Mos̈ DM, Araszkiewicz A, Szromek AR. Serum concentrations of chemokines (CCL-5 and CXCL-12), chemokine receptors (CCR-5 and CXCR-4), and IL-6 in patients with posttraumatic stress disorder and avoidant personality disorder. Pharmacological Reports. 2015;67:1251–1258. - PubMed
-
- Ozawa PMM, Ariza CB, Ishibashi CM, Fujita TC, Banin-Hirata BK, Oda JMM, et al. Role of CXCL12 and CXCR4 in normal cerebellar development and medulloblastoma. International Journal of Cancer. 2016;138:10–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

