Downregulation of thrombospondin-1 by DNA hypermethylation is associated with tumor progression in laryngeal squamous cell carcinoma
- PMID: 27485791
- PMCID: PMC4991671
- DOI: 10.3892/mmr.2016.5580
Downregulation of thrombospondin-1 by DNA hypermethylation is associated with tumor progression in laryngeal squamous cell carcinoma
Abstract
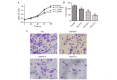
Thrombospondin‑1 (THBS‑1) has been demonstrated to have a complicated role in human cancer and to exert stimulatory and inhibitory effects in different types of tumors. DNA methylation, as the most frequent mechanism for gene silencing, has been widely investigated in regards to the development of tumors. However, the expression levels and methylation status of THBS‑1, and their roles in laryngeal squamous cell carcinoma (LSCC) remain to be elucidated. The present study detected downregulated THBS‑1 mRNA and protein expression levels in LSCC by using reverse transcription-quantitative polymerase chain reaction (PCR) and western blotting, while decreased expression levels of THBS‑1 mRNA and protein were significantly associated with lymph node metastasis and tumor‑node‑metastasis (TNM) stage. Furthermore, aberrant methylation of THBS‑1 was frequently observed in LSCC by methylation‑specific PCR, particularly in tumor tissues from lymph node metastasis or samples from cancer with advanced TNM stage. Furthermore, the current study demonstrated that downregulated expression of THBS‑1 in LSCC was consistent with aberrant methylation of this gene. Treatment with the DNA methyltransferase inhibitor 5-aza-2'-deoxy-cytidine in Hep‑2 cells induced demethylation of THBS-1, enhanced THBS‑1 expression, and inhibited the proliferative and invasive ability of Hep‑2 cells. Collectively, the results of the present study suggest that THBS‑1 may exert an inhibitory effect in the development of LSCC. Aberrant methylation was an important reason for the downregulation of THBS‑1 and was involved in the invasion and metastasis of LSCC. Demethylating agents may be effective candidates for the treatment of LSCC.
Figures


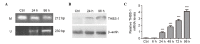
Similar articles
-
Methylation-associated silencing of death-associated protein kinase gene in laryngeal squamous cell cancer.Laryngoscope. 2005 Aug;115(8):1395-401. doi: 10.1097/01.MLG.0000166708.23673.3A. Laryngoscope. 2005. PMID: 16094112
-
Aberrant methylation‑mediated decrease of lncRNA HNF1A‑AS1 contributes to malignant progression of laryngeal squamous cell carcinoma via EMT.Oncol Rep. 2020 Dec;44(6):2503-2516. doi: 10.3892/or.2020.7823. Epub 2020 Oct 23. Oncol Rep. 2020. PMID: 33125127 Free PMC article.
-
[Study of mRNA expression level and hypermethylation of CHFR promoter in the laryngeal squamous cell carcinoma tissue].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010 Aug;24(15):673-7. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010. PMID: 20942233 Chinese.
-
[Progress in research on DNA methylation and laryngeal carcinoma].Zhonghua Bing Li Xue Za Zhi. 2011 Jan;40(1):67-70. Zhonghua Bing Li Xue Za Zhi. 2011. PMID: 21429368 Review. Chinese. No abstract available.
-
A Novel ALK-THBS1 Fusion in a Laryngeal Inflammatory Myofibroblastic Tumour: A Case Report and Literature Review.Head Neck Pathol. 2020 Jun;14(2):454-458. doi: 10.1007/s12105-019-01061-x. Epub 2019 Jul 31. Head Neck Pathol. 2020. PMID: 31368077 Free PMC article. Review.
Cited by
-
Circulating methylated THBS1 DNAs as a novel marker for predicting peritoneal dissemination in gastric cancer.J Clin Lab Anal. 2021 Sep;35(9):e23936. doi: 10.1002/jcla.23936. Epub 2021 Aug 13. J Clin Lab Anal. 2021. PMID: 34390026 Free PMC article.
-
Long non-coding RNA BZRAP1-AS1 silencing suppresses tumor angiogenesis in hepatocellular carcinoma by mediating THBS1 methylation.J Transl Med. 2019 Dec 17;17(1):421. doi: 10.1186/s12967-019-02145-6. J Transl Med. 2019. PMID: 31847842 Free PMC article.
-
MicroRNA-3907 promotes the proliferation and migration of sebaceous gland carcinoma of the eyelid by targeting thrombospondin 1.Oncol Lett. 2021 Dec;22(6):833. doi: 10.3892/ol.2021.13094. Epub 2021 Oct 14. Oncol Lett. 2021. PMID: 34691259 Free PMC article.
-
SWI/SNF ATPase silenced HLF potentiates lung metastasis in solid cancers.Nat Commun. 2025 Jun 5;16(1):5226. doi: 10.1038/s41467-025-60329-9. Nat Commun. 2025. PMID: 40473600 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

