Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation
- PMID: 27485817
- PMCID: PMC4971566
- DOI: 10.1038/srep31042
Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation
Abstract
Controversy exists regarding whether left atrial enlargement (LAE) is a predictor of stroke/systemic embolism (SE) in atrial fibrillation (AF) patients. The Fushimi AF Registry, a community-based prospective survey, enrolled all AF patients in Fushmi-ku, Japan, from March 2011. Follow-up data and baseline echocardiographic data were available for 2,713 patients by August 2015. We compared backgrounds and incidence of events over a median follow-up of 976.5 days between patients with LAE (left atrial diameter > 45 mm; LAE group) and those without in the Fushimi AF Registry. The LAE group accounted for 39% (n = 1,049) of cohort. The LAE group was older and had longer AF duration, with more prevalent non-paroxysmal AF, higher CHADS2/CHA2DS2-VASc score, and oral anticoagulant (OAC) use. A higher risk of stroke/SE during follow-up in the LAE group was found (entire cohort; hazard ratio (HR): 1.92, 95% confidence interval (CI): 1.40-2.64; p < 0.01; without OAC; HR: 1.97, 95% CI: 1.18-3.25; p < 0.01; with OAC; HR: 1.83, 95% CI: 1.21-2.82; p < 0.01). LAE was independently associated with increased risk of stroke/SE (HR: 1.74, 95% CI: 1.25-2.42; p < 0.01) after adjustment by the components of CHA2DS2-VASc score and OAC use. In conclusion, LAE was an independent predictor of stroke/SE in large community cohort of AF patients.
Conflict of interest statement
Dr. Akao received lecture fees from Pfizer, Bristol Myers Squibb, Boehringer Ingelheim, Bayer Healthcare and Daiichi-Sankyo. Dr Lip has served as a consultant or advisory board for Bayer/ Janssen, Astellas, Merck, Sanofi, BMS/Pfizer, Biotronik, Medtronic, Portola, Boehringer Ingelheim, Microlife and Daiichi-Sankyo, and has been on the speakers bureau for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche and Daiichi-Sankyo. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



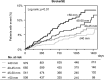
References
-
- Wolf P. A., Abbott R. D. & Kannel W. B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22, 983–988 (1991). - PubMed
-
- Pisters R., Lane D. A., Marin F., Camm A. J. & Lip G. Y. Stroke and thromboembolism in atrial fibrillation. Circ J 76, 2289–2304 (2012). - PubMed
-
- Gage B. F. et al.. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285, 2864–2870 (2001). - PubMed
-
- Lip G. Y., Nieuwlaat R., Pisters R., Lane D. A. & Crijns H. J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137, 263–272, 10.1378/chest.09-1584 (2010). - DOI - PubMed
-
- Manning W. J., Silverman D. I., Keighley C. S., Oettgen P. & Douglas P. S. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short-term anticoagulation: final results of a prospective 4.5-year study. J Am Coll Cardiol 25, 1354–1361, 10.1016/0735-1097(94)00560-d (1995). - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

