Structural insights into the catalysis and regulation of E3 ubiquitin ligases
- PMID: 27485899
- PMCID: PMC6211636
- DOI: 10.1038/nrm.2016.91
Structural insights into the catalysis and regulation of E3 ubiquitin ligases
Abstract
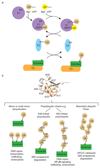
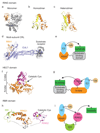
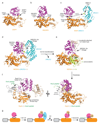
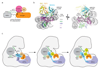
Covalent attachment (conjugation) of one or more ubiquitin molecules to protein substrates governs numerous eukaryotic cellular processes, including apoptosis, cell division and immune responses. Ubiquitylation was originally associated with protein degradation, but it is now clear that ubiquitylation also mediates processes such as protein-protein interactions and cell signalling depending on the type of ubiquitin conjugation. Ubiquitin ligases (E3s) catalyse the final step of ubiquitin conjugation by transferring ubiquitin from ubiquitin-conjugating enzymes (E2s) to substrates. In humans, more than 600 E3s contribute to determining the fates of thousands of substrates; hence, E3s need to be tightly regulated to ensure accurate substrate ubiquitylation. Recent findings illustrate how E3s function on a structural level and how they coordinate with E2s and substrates to meticulously conjugate ubiquitin. Insights regarding the mechanisms of E3 regulation, including structural aspects of their autoinhibition and activation are also emerging.
Figures



References
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. - PubMed
-
- Dye BT, Schulman BA. Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu Rev Biophys Biomol Struct. 2007;36:131–50. - PubMed
-
- Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695:55–72. - PubMed
-
- Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–29. - PubMed
-
- Chau V, et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

