Velocity dependence of vestibular information for postural control on tilting surfaces
- PMID: 27486101
- PMCID: PMC5040378
- DOI: 10.1152/jn.00057.2016
Velocity dependence of vestibular information for postural control on tilting surfaces
Abstract
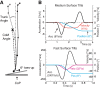
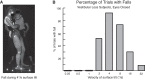
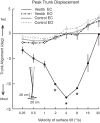
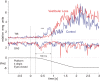
Vestibular information is known to be important for postural stability on tilting surfaces, but the relative importance of vestibular information across a wide range of surface tilt velocities is less clear. We compared how tilt velocity influences postural orientation and stability in nine subjects with bilateral vestibular loss and nine age-matched, control subjects. Subjects stood on a force platform that tilted 6 deg, toes-up at eight velocities (0.25 to 32 deg/s), with and without vision. Results showed that visual information effectively compensated for lack of vestibular information at all tilt velocities. However, with eyes closed, subjects with vestibular loss were most unstable within a critical tilt velocity range of 2 to 8 deg/s. Subjects with vestibular deficiency lost their balance in more than 90% of trials during the 4 deg/s condition, but never fell during slower tilts (0.25-1 deg/s) and fell only very rarely during faster tilts (16-32 deg/s). At the critical velocity range in which falls occurred, the body center of mass stayed aligned with respect to the surface, onset of ankle dorsiflexion was delayed, and there was delayed or absent gastrocnemius inhibition, suggesting that subjects were attempting to actively align their upper bodies with respect to the moving surface instead of to gravity. Vestibular information may be critical for stability at velocities of 2 to 8 deg/s because postural sway above 2 deg/s may be too fast to elicit stabilizing responses through the graviceptive somatosensory system, and postural sway below 8 deg/s may be too slow for somatosensory-triggered responses or passive stabilization from trunk inertia.
Keywords: human; postural stability; proprioception; somatosensory; surface tilt; vestibular.
Copyright © 2016 the American Physiological Society.
Figures




References
-
- Allum JH, Pfaltz CR. Visual and vestibular contributions to pitch sway stabilization in the ankle muscles of normals and patients with bilateral peripheral vestibular deficits. Exp Brain Res 58: 82–94, 1985. - PubMed
-
- Allum JH, Tang KS, Carpenter MG, Oude Nijhuis LB, Bloem BR. Review of first trial responses in balance control: influence of vestibular loss and Parkinson's disease. Hum Mov Sci 30: 279–295, 2011. - PubMed
-
- Buchanan JJ, Horak FB. Emergence of postural patterns as a function of vision and translation frequency. J Neurophysiol 81: 2325–2339, 1999. - PubMed
-
- Buchanan JJ, Horak FB. Vestibular loss disrupts control of head and trunk on a sinusoidally moving platform. J Vestib Res 11: 371–389, 2001. - PubMed
-
- Carpenter MG, Allum JH, Honegger F. Vestibular influences on human postural control in combinations of pitch and roll planes reveal differences in spatiotemporal processing. Exp Brain Res 140: 95–111, 2001. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

