Firing characteristics of deep dorsal horn neurons after acute spinal transection during administration of agonists for 5-HT1B/1D and NMDA receptors
- PMID: 27486104
- PMCID: PMC5144700
- DOI: 10.1152/jn.00198.2016
Firing characteristics of deep dorsal horn neurons after acute spinal transection during administration of agonists for 5-HT1B/1D and NMDA receptors
Abstract
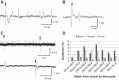
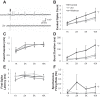
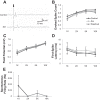
Spinal cord injury (SCI) results in a loss of serotonin (5-HT) to the spinal cord and a loss of inhibition to deep dorsal horn (DDH) neurons, which produces an exaggerated excitatory drive to motoneurons. The mechanism of this excitatory drive could involve the DDH neurons triggering long excitatory postsynaptic potentials in motoneurons, which may ultimately drive muscle spasms. Modifying the activity of DDH neurons with drugs such as NMDA or the 5-HT1B/1D receptor agonist zolmitriptan could have a large effect on motoneuron activity and, therefore, on muscle spasms. In this study, we characterize the firing properties of DDH neurons after acute spinal transection in adult mice during administration of zolmitriptan and NMDA, using the in vitro sacral cord preparation and extracellular electrophysiology. DDH neurons can be categorized into three major types with distinct evoked and spontaneous firing characteristics: burst (bursting), simple (single spiking), and tonic (spontaneously tonic firing) neurons. The burst neurons likely contribute to muscle spasm mechanisms because of their bursting behavior. Only the burst neurons show significant changes in their firing characteristics during zolmitriptan and NMDA administration. Zolmitriptan suppresses the burst neurons by reducing their evoked spikes, burst duration, and spontaneous firing rate. Conversely, NMDA facilitates them by enhancing their burst duration and spontaneous firing rate. These results suggest that zolmitriptan may exert its antispastic effect on the burst neurons via activation of 5-HT1B/1D receptors, whereas activation of NMDA receptors may facilitate the burst neurons in contributing to muscle spasm mechanisms following SCI.
Keywords: N-methyl-d-aspartate; deep dorsal horn neurons; serotonin; spinal cord injury.
Copyright © 2016 the American Physiological Society.
Figures



Comment in
-
Bursting deep dorsal horn neurons: the pharmacological target for the antispastic effects of zolmitriptan?J Neurophysiol. 2017 May 1;117(5):1841-1843. doi: 10.1152/jn.00880.2016. Epub 2016 Dec 14. J Neurophysiol. 2017. PMID: 27974452 Free PMC article.
Similar articles
-
Bursting deep dorsal horn neurons: the pharmacological target for the antispastic effects of zolmitriptan?J Neurophysiol. 2017 May 1;117(5):1841-1843. doi: 10.1152/jn.00880.2016. Epub 2016 Dec 14. J Neurophysiol. 2017. PMID: 27974452 Free PMC article.
-
Bursting interneurons in the deep dorsal horn develop increased excitability and sensitivity to serotonin after chronic spinal injury.J Neurophysiol. 2020 May 1;123(5):1657-1670. doi: 10.1152/jn.00701.2019. Epub 2020 Mar 25. J Neurophysiol. 2020. PMID: 32208883 Free PMC article.
-
Reduction of spinal sensory transmission by facilitation of 5-HT1B/D receptors in noninjured and spinal cord-injured humans.J Neurophysiol. 2013 Mar;109(6):1485-93. doi: 10.1152/jn.00822.2012. Epub 2012 Dec 5. J Neurophysiol. 2013. PMID: 23221401 Free PMC article. Clinical Trial.
-
Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5-HT1B and 5-HT1F receptors.J Neurophysiol. 2011 Aug;106(2):925-43. doi: 10.1152/jn.01011.2010. Epub 2011 Jun 8. J Neurophysiol. 2011. PMID: 21653728 Free PMC article.
-
Noise or signal? Spontaneous activity of dorsal horn neurons: patterns and function in health and disease.Pflugers Arch. 2024 Aug;476(8):1171-1186. doi: 10.1007/s00424-024-02971-8. Epub 2024 Jun 1. Pflugers Arch. 2024. PMID: 38822875 Free PMC article. Review.
Cited by
-
Bursting deep dorsal horn neurons: the pharmacological target for the antispastic effects of zolmitriptan?J Neurophysiol. 2017 May 1;117(5):1841-1843. doi: 10.1152/jn.00880.2016. Epub 2016 Dec 14. J Neurophysiol. 2017. PMID: 27974452 Free PMC article.
-
Bursting interneurons in the deep dorsal horn develop increased excitability and sensitivity to serotonin after chronic spinal injury.J Neurophysiol. 2020 May 1;123(5):1657-1670. doi: 10.1152/jn.00701.2019. Epub 2020 Mar 25. J Neurophysiol. 2020. PMID: 32208883 Free PMC article.
-
The Involvement of CaV1.3 Channels in Prolonged Root Reflexes and Its Potential as a Therapeutic Target in Spinal Cord Injury.Front Neural Circuits. 2021 Mar 23;15:642111. doi: 10.3389/fncir.2021.642111. eCollection 2021. Front Neural Circuits. 2021. PMID: 33867945 Free PMC article.
-
An electrophysiologist's guide to dorsal horn excitability and pain.Front Cell Neurosci. 2025 Apr 2;19:1548252. doi: 10.3389/fncel.2025.1548252. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40241846 Free PMC article. Review.
-
Spontaneous Multimodal Neural Transmission Suggests That Adult Spinal Networks Maintain an Intrinsic State of Readiness to Execute Sensorimotor Behaviors.J Neurosci. 2021 Sep 22;41(38):7978-7990. doi: 10.1523/JNEUROSCI.0662-21.2021. Epub 2021 Aug 11. J Neurosci. 2021. PMID: 34380765 Free PMC article.
References
-
- Baker LL, Chandler SH. Characterization of postsynaptic potentials evoked by sural nerve stimulation in hindlimb motoneurons from acute and chronic spinal cats. Brain Res 420: 340–350, 1987. - PubMed
-
- Bennett DJ, Sanelli L, Cooke C, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol 91: 2247–2258, 2004. - PubMed
-
- Blanke ML, VanDongen AM. Activation mechanisms of the NMDA receptor. In: Biology of the NMDA Receptor, edited by VanDongen AM. Boca Raton, FL: CRC/Taylor & Francis, 2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

